Gabriel F. Pozo de Mattos P., Basic Health Sciences Department, Federal University of Health Sciences of Porto Alegre (UFCSPA), Immunotherapy Laboratory-UFCSPA, R. Sarmento Leite, 245-Centro Histórico, Porto Alegre, 90050-170, Brazil. E-mail: gabriel13mattos@gmail.com
Abstract
Aims: Aged individuals are significantly underrepresented in immunotherapy clinical trials for cancer. Little is known regarding the immunological and molecular dynamics that might regulate their responsiveness to immune checkpoint inhibitors (ICIs). This study aims to investigate the mechanisms affecting the response of elderly non-small cell lung cancer (NSCLC) patients to anti-PD-1 therapy.
Methods: We performed a single-cell analysis on public data from 419,107 tumor-infiltrating lymphocytes (TILs) across 11 elderly (≥ 65 years) and 5 non-elderly NSCLC patients treated with neoadjuvant anti-PD-1 therapy. The dataset, originally focused on mutation-associated neoantigen-specific T cells, was reprocessed to compare gene expression and molecular patterns associated with positive outcomes and tumor clearance between age groups.
Results: The analysis revealed that ICI responsiveness was not impaired by age, and T cell immunosenescence was observed in aged (≥ 65 years) and younger NSCLC individuals. Both elderly and young individuals produced responses with a heterogeneous molecular program associated with tumor-reactive CD8+ TILs. Specifically, T cells from elderly patients showed an enhanced expression of PDCD1 and CXCL13 (P < 0.001) in comparison to younger subjects.
Conclusion: Altogether, our findings demonstrate favorable molecular signatures in aged NSCLC individuals following anti-PD-1 treatment and suggest that the recruitment of older adults in immunotherapy clinical trials should not be dismissed solely on the grounds of age.
Keywords
1. Introduction
Cancer is a multifactorial disease consisting of several inherited and epigenetic changes in the physiological state of a cell. Its hallmarks include sustained proliferative signaling, cell death resistance, and replicative immortality[1]. Such hallmarks are rooted in genomic instability and cellular damage accumulation, which are time-dependent. It is thus not surprising that most tumors correlate with chronological aging and exhibit a higher prevalence in the elderly population[2-4].
In addition to age-related modifications, epigenetic and environmental modulators during life contribute to a parallel phenomenon known as senescence. At the molecular and cellular levels, senescence describes a downregulation in function and cell division potential. Immunosenescence describes functional alterations observed in immune cells and is mostly thought of as restricted to the elderly[5]. Aging and senescence are often quoted as synonyms, and yet, despite being associated with aging, senescence does not exclusively depend on time[6]. For example, senescent cells and genetic signatures associated with senescence are frequent in the tumor microenvironment (TME) of individuals of all ages[7].
Immunosenescence is one important reason for excluding aged patients from immune checkpoint inhibitors (ICI) clinical trials. The prediction is that they will not respond to treatment that recovers anti-tumor T cell function because their T cells are already compromised beyond help. However, clinical reports show that older individuals tend to benefit more from cancer immunotherapy with ICIs than younger subjects[8-11], highlighting an important gap between age-related ICI responsiveness and its underlying molecular mechanisms.
Here, we sought to identify molecular patterns that associate with response to ICI in aged individuals. First, we found an expressive underrepresentation of aged individuals in clinical trials using anti-tumoral immunotherapy[12,13-16], making it difficult to gather representative data for a robust metanalysis. We next searched 334 public datasets to identify the ones which included aged patients for oncologic immunotherapy. Of those, the study of Caushi et al.[17] was selected for a more detailed examination because it included a relatively large number of aged and young patients with non-small cell lung cancer (NSCLC), all receiving the same treatment-neoadjuvant anti-PD-1. In that study, the authors analyzed exclusively CD3+ cells from the TME, draining lymph nodes and adjacent tissue investigating the clonality of anti-tumor T cell responses. We thus chose and reanalyzed the Caushi et al.[17] single-cell RNA-seq data of tumor-infiltrating T cells in elderly and non-elderly patients, now focusing on identifying gene expression and molecular patterns associated with positive outcomes and tumor clearance in aged individuals. The figures in this manuscript illustrate the all-new information generated by our analysis. We found that therapy responsiveness is achieved despite age, although T cell immunosenescence occurs both in aged and younger NSCLC individuals. Our results indicate that treatment of aged cancer patients with ICIs should not be dismissed a priori and reveals that both young and elderly can produce CD8+ T cells with genetic signatures that associate with anti-tumor responses.
2. Methods
2.1 Dataset search
First, we searched for public single-cell datasets of patients who received neoadjuvant immunotherapy because it has recently been reported that long-lasting anti-tumor immune responses in NSCLC patients are achieved with this treatment[18] and it now constitutes a trend in immunotherapy. We performed the dataset search in IMMUcan SingleCell RNAseq Database[19] and TISCH2[20], two public repositories composed of available single-cell RNA-sequencing data from original publications. Our key inclusion criteria filtered datasets contemplating single-cell sequencing analysis of tumor-infiltrating T cells of cancer patients under and above 65 years old, with the same tumor type, and undergoing the same treatment. We constructed a table with the public cancer datasets that fit our inclusion criteria (Table S1). Only 7 (out of a total of 334) contained enough patients with the treatment protocols that included immunotherapy. In six of them, there was more than one treatment, and the number of patients had to be divided; in half of them, the age of the patients had not been disclosed; and 3 of them did not have a high number of cells analyzed. These six datasets were thus excluded. We analyzed the clinical metadata available (so we could split the patients into two groups: elderly and non-elderly) and the total number of cells. GSE176021, from the work of Caushi et al.[17], was the only one that filled our criteria. Compared to other datasets, this work gathered a sufficient number of elderly and non-elderly patients to perform a comparison between groups, besides the high quantity of T cells analyzed (more than 400,000). Patients under 65 years old were classified as non-elderly, whilst patients equal to or above 65 years-old were classified as elderly. We conducted a reanalysis of these previously published scRNA-seq data.
The dataset consists of 16 lung cancer patients who received neoadjuvant anti-PD-1 and underwent resection afterward. The authors applied FACS before sequencing to retain only CD3-positive cells. We performed clusterization, cell type annotation and identified gene modules associated with functional roles in tumor-infiltrating lymphocytes from patients’ biopsies after resection. To ensure a rich amount of T cells for the analysis (419,107 counts), patients had more than one sample collected through a standardized process, and following comparisons were performed between patients.
2.2 Patients
NSCLC patients (stages I, II, or IIIA ≥ 18 years old) candidates for tumor resection were enrolled, as previously described[17]. Briefly, all patients had normal organ and pulmonary functions. Patients with any immunodeficiency, ongoing systemic immunosuppressive therapy, active autoimmune or infectious disease, and clinically significant concurrent cancer were not included. Regarding the study design, this single-group study was conducted at two medical centers in the United States. The patients received two doses of intravenous nivolumab (3 mg per kilogram of body weight) every 2 weeks. Surgery was planned to be performed approximately 4 weeks after the first dose. Table S2 and Table S3 comprise all the collection processes and histological features for each patient, respectively.
2.3 Ethical approval
All ethical disclosures regarding patients’ inclusion and ethics statements (ClinicalTrials.gov ID NCT02259621) can be obtained from the original publication[17] where we stem the data.
2.4 Data download, processing, and filtering
Data was obtained from GEO database code accession GSE176021. Processing and filtering were performed in Seurat. Cells with less than 1,000 nCount_RNA and more than 100,000; expressing less than 500 genes and more than 10,000, more than 30% mitochondrial counts and 5% hemoglobin counts were filtered out.
2.5 Data integration and cluster annotation
We imported the counts’ matrix in Scanpy and performed batch correction and integration with scVI-tools[21]. First, we subsetted the counts’ matrix to retain only the top 3000 HVG (highly-variable genes). Afterward, we defined our batch as ‘sample_id’ and trained our model with the following hyperparameters: n_layers = 2, n_latent = 30, dropout_rate = 0.1. After training the model and performing batch correction, we clustered our data using the Leiden algorithm with a resolution of 3.
Then, we extracted the latent space from our model and projected it to a 2D space using Uniform Manifold Approximation and Projection (UMAP). Before cell type annotation, we removed 3 small clusters with low-quality counts. Canonical gene markers were used to classify our cells as CD4, CD4-Treg, CD8, and MAIT in this first-level annotation. For a better characterization of CD4 and CD8 cells, we isolated them and reclustered them separately using the same parameters (just a slight change in the HVG selection; n = 2,000 instead of 3,000 previously) for the model training. To assess gene markers for CD4 and CD8 subsets, we applied differential gene expression using the function ‘sc.tl.rank_genes_groups (method = wilcoxon)’.
2.6 Molecular programs, PCA, plots and statistics
In order to refine our CD8 T classification based on molecular programs, we used the Hotspot package. Hotspot performs feature selection as follows: genes with significant local autocorrelation are grouped into modules based on co-expression between nearby cells in the similarity graph. We used the 2,000 HVG and retained only the top 500 genes with the highest autocorrelation.
For module score comparison between groups (MPR vs. non-MPR; Elderly vs. Non-elderly), we aggregated the scores by patient and extracted the mean for each module. Plots were generated with ggplot2 and wilcoxon test was performed with ggpubr.
To build the PCA, anndata object was converted to SingleCellExperiment object. Counts were aggregated by patient. This matrix was used as input for Deseq2 object[22]. Finally, we called the function plotPCA to see the plot. Genes that explain each PC are detailed in Table S4. For Figure 1G,H, we could not perform a statistical analysis. In a single-cell level, different proportions in cell subsets may lead to impaired test precision and false-positive results. Bar plots were constructed just for visualization purposes. For Table 1, the normality of values was calculated by the Shapiro-Wilk test. Statistical analysis with unpaired T-test, Fisher’s Exact test and the non-parametric Mann-Whitney test, with P-values < 0.05 (*), < 0.01 (**) and < 0.001 (***).
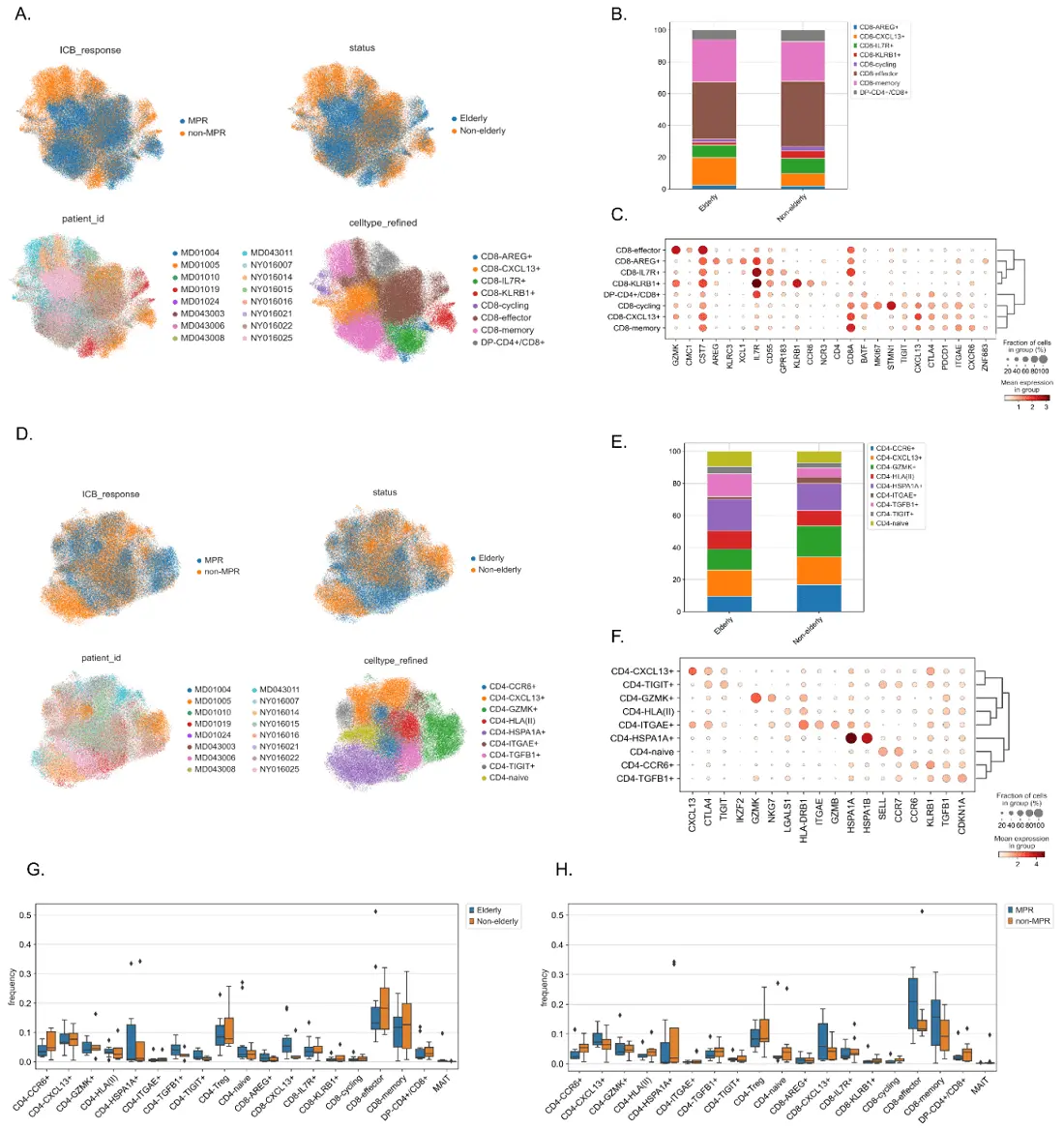
Figure 1. CD4+ and CD8+ T cell clustering according to gene profiles in NSCLC patients following anti-PD-1. (A-C) CD4+ and CD8+ clustering through functional markers. (A) UMAPs projecting CD8+ T cell clustering into eight subsets (lower right), according to age group (upper right) and response to treatment (upper left); (B) CD8+ subset proportions between elderly (n = 11) and non-elderly (n = 5) patients. Each cluster was constructed based on the dot-plot of gene expression (C), with color and size representing positive expressions; (D) UMAPs projecting CD4+ T cell clustering into nine subsets (lower right), according to age group (upper right) and response to treatment (upper left); (E) CD4+ subset proportions between elderly (n = 11) and non-elderly (n = 5) patients. Each cluster was constructed based on the dot-plot of gene expression (F), with color and size representing positive expressions; (G) Bar plot of CD4+ and CD8+ T cell subsets in elderly (n = 11, blue plot) and non-elderly (n = 5, orange plot) NSCLC patients following anti-PD-1 treatment. Bars designate median with IQR; (H) Bar plot of CD4+ and CD8+ T cell subsets in MPR (n = 7, blue plot) and non-MPR (n = 9), orange plot) NSCLC patients following anti-PD-1 treatment. Lozenges represent outliers. Bars designate median with IQR; (G-H) Statistical analysis could not be performed. NSCLC: non-small cell lung cancer; UMAP: uniform manifold approximation and projection; IQR: interquartile range; MPR: major pathologic response; ICB: immune checkpoint blockade.
| Elderly (≥ 65 years) | Non-elderly (< 65 years) | P-value | |
| N | 11 | 5 | |
| Demographic data | |||
| Age at diagnosis, mean ± SD | 71.5 ± 4.3 | 58.8 ± 2.8 | *** $ |
| Female, n (%) | 7 (63.6) | 2 (40) | ns& |
| Histology, n (%) | |||
| Squamous Cell Carcinoma | 3 (27.3) | 1 (20) | ns& |
| Adenosquamous | 1 (9) | 0 | |
| Adenocarcinoma | 7 (63.6) | 4 (80) | |
| ICI response, n (%) | |||
| Responsive to treatment | 5 (45.4) | 2 (40) | ns& |
| % residual tumor, median (IQR) | 40 (5-95) | 75 (2.5-87.5) | ns# |
Normality of values calculated by Shapiro-Wilk test. Statistical analysis with ($) unpaired T-test, (&) Fisher's Exact test and (#) the non-parametric Mann-Whitney test, with P-values < 0.05 (*), < 0.01 (**) and < 0.001 (***). IQR: interquartile range.
3. Results
3.1 Elderly and non-elderly NSCLC patients treated with anti-PD-1 exhibit comparable immunosenescence features
We sought to characterize the tumor-infiltrating T cell compartment of elderly (≥ 65 years) and non-elderly patients treated with neoadjuvant anti-PD-1 (nivolumab) in a resectable NSCLC model. These data stem from the work of Caushi and colleagues[17] and provided us with the single-cell analysis of 16 NSCLC patients treated with anti-PD-1 prior to surgery, 11 of them with ages above or equal to 65 years, as presented in Table 1.
Response to immunotherapy was evaluated by major pathologic response (MPR), defined as residual viable tumor ≤ 10%[17]. Five out of 11 (45.4%) aged patients were responsive to treatment in comparison to two out of five (40%) in the non-elderly group, which also displayed a 75% median percentage of residual tumor versus 40% in elderly patients. Given the small sample size and elevated range, this analysis was not statistically significant.
We first sought to characterize major T cell populations across all patients. The single-cell analysis was performed in Scanpy and provided a total of 419,107 T cells from tumor specimens (Figure 2A). Subsequent clustering revealed 202,384 CD8+, 168,314 CD4+, 43,153 CD4-Treg, and 5,256 MAIT cells (Figure 2B and Figure S1A; differentially expressed genes for all cells are present in Table S5). Interestingly, elderly and non-elderly individuals displayed an overall similar proportion of these populations as shown in Figure 2C,D. These results reveal a similar cell type composition between elderly and non-elderly patients.
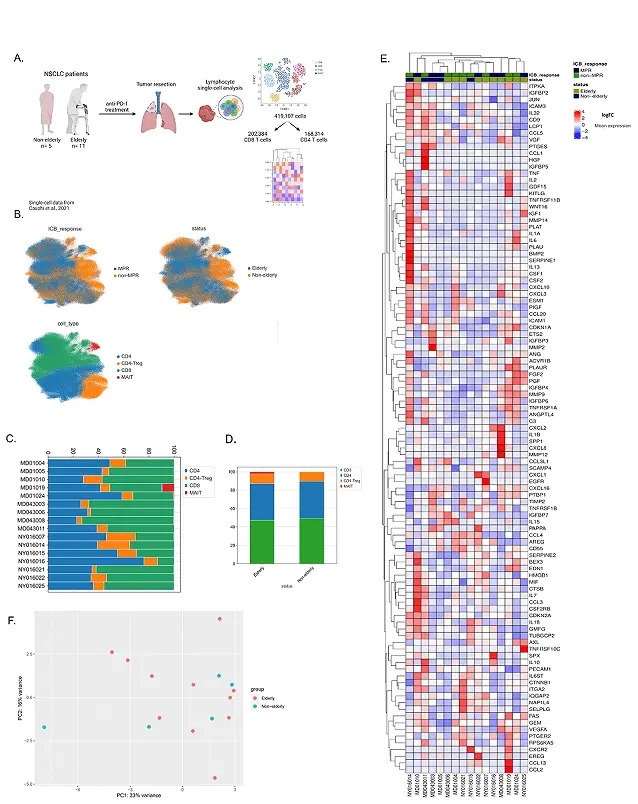
Figure 2. T cell populations and immunosenescence features in elderly and non-elderly NSCLC patients treated with anti-PD-1. (A) Schematic representation of the study design (created with Biorender); (B) T cell subpopulations clustered by specific markers: UMAPs representing T cell subsets (lower left): CD8+, CD4+, Tregs and MAIT T cells; age group (upper right); and response to anti-PD-1 (upper left); (C-D) Column graph with T cell proportions; (C) across each patient; and (D) among elderly (n = 11) and non-elderly (n = 5) patients; (E) Immunosenescence genes expressed in NSCLC individuals: heatmap with senescence-related genes among elderly vs. non-elderly patients, and MPR vs. non-MPR individuals. Genes designate each row, whereas columns are representative of each patient. The median expression is expressed as high (red) or low/negative (blue); (F) Clustering of NSCLC patients based on immunosenescence genes: PCA of senescence-related genes from elderly and non-elderly NSCLC patients treated with anti-PD-1. NSCLC: non-small cell lung cancer; MPR: major pathologic response; PCA: principal component analysis.
We next asked if senescence-related genes would be more expressed in elderly or non-elderly ICI-treated patients. Using SenMayo gene set[23], a database composed of senescence-related markers, we compared the expression of 103 genes (Table S6) considering age and response to immunotherapy. The heatmap in Figure 2E showed an overall enrichment of senescence markers in all patients, with no obvious pattern of expression associated with age or therapy response. Of note, the most pronounced upregulation of senescence genes belonged to a non-MPR patient in the non-elderly group (NY016014), which also exhibited the highest proportion of Tregs in the TME (Figure 2C). This may reflect a Treg counter-regulation mechanism to restrain the excess of proinflammatory mediators provoked by SASP-producing cells, contributing to the observed unresponsiveness to immunotherapy.
We then performed a PCA in order to visualize if elderly and non-elderly adults would differ based on the same genes (Table S4) used in the heatmap. With 50% of the variance explained by PCAs 1 and 2, senescence genes were not able to distinguish elderly from non-elderly T cells (Figure 2F and Figure S1B). Using different colors identifying MPR and non-MPR individuals, the PCA also did not evidence any specific pattern related to therapy responsiveness (Figure S1C). Altogether, our findings indicate that immunosenescence is not age-dependent in a tumoral context and does not impair the response to anti-PD-1 in NSCLC patients.
3.2 Aged individuals share similar CD4+ and CD8+ populations in the TME compared to the non-elderly
We asked if the elderly could harbor different T cell profiles compared to the non-elderly. To do that, we reclustered T cells to better characterize CD4+ and CD8+ subtypes. Figure 1A depicts the CD8+ clustering giving rise to eight subpopulations, whilst the CD4+ refinement converged to nine subpopulations (Figure 1D; differentially expressed genes for CD4 and CD8 subsets are present in Table S7 and Table S8). Both of them were annotated based on the dot-plot gene expression shown in Figure 1C,D,E,F. Further comparison between groups revealed an elevated proportion of CD8+CXCL13+ and CD4+TGFB1+ T cells in the elderly (Figure 1B,E,G, Figure S1D,E). We also investigated if these subsets had any differences according to response to anti-PD-1, as shown in Figure 1H, and observed that MPR-individuals tended to have higher proportions of CD8+CXCL13+, CD8-effector and CD8-memory populations.
We then investigated specific molecular programs in the CD8+ clusters to establish their impact in age and therapy efficacy. Using the Hotspot package[24], we identified 16 phenotypes or modules (genes enriched for each module can be found in Table S9) originated from positive correlations between genes (Figure 3A,C). This clustering resulted in the characterization of previously described CD8+ profiles associated with cytotoxic response (module 15), stemness (module 12) and memory (module 4 and module 13) (Figure 3B,D). Clusters 6 and 8 fit well established phenotypes of early tumor effector memory and ‘dysfunctional-like’/tumor-reactive cells, both associated with immunotherapy responsiveness[25,26]. Interestingly, these molecular programs were enriched in the same UMAP areas as CD8-effectors, CD8-memory and CD8+CXCL13+ populations, all three visually enhanced in MPR patients. Additional modules were representative of stressed (1 and 2) and cycling cells (3).
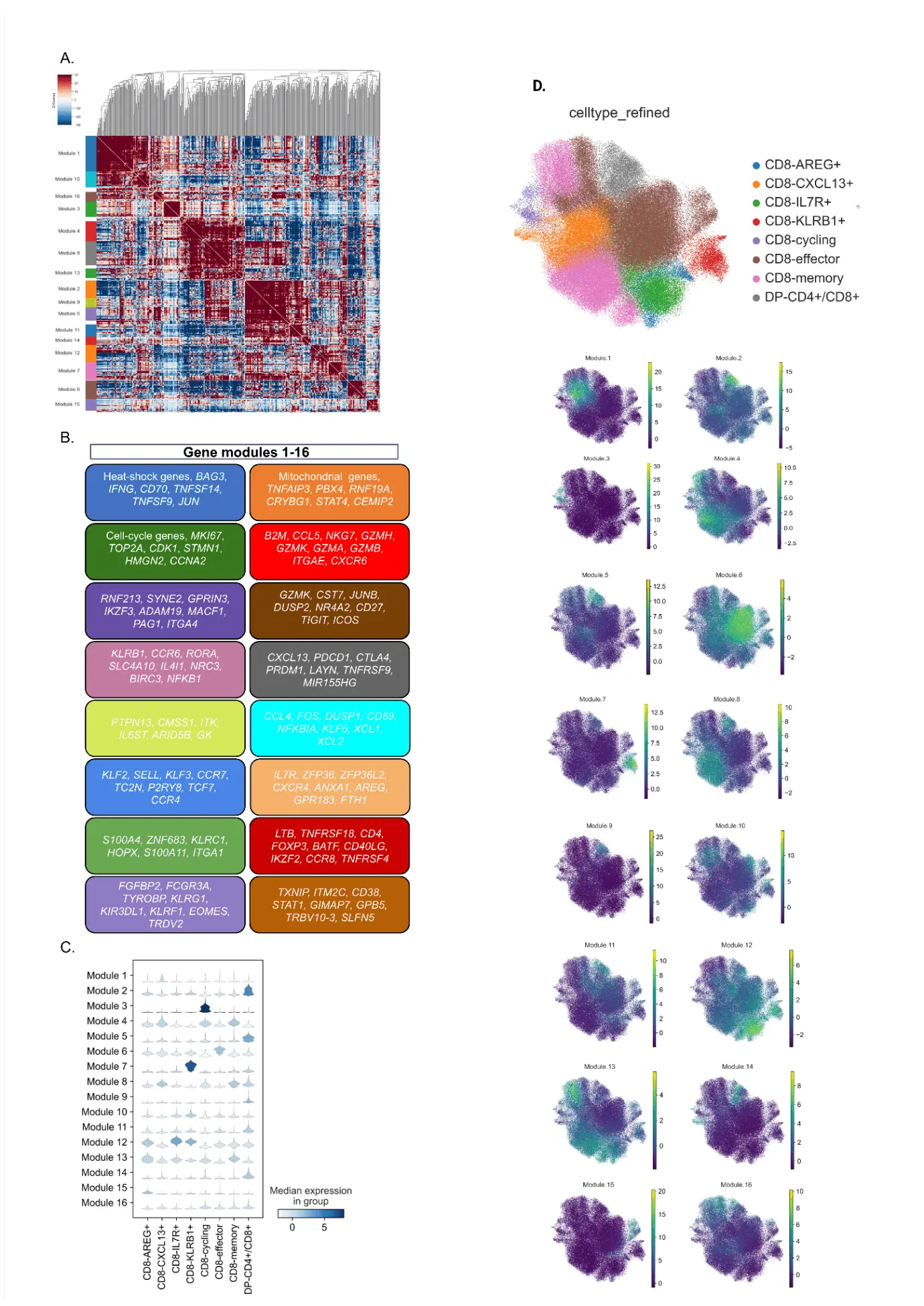
Figure 3. Molecular programs in CD8+ tumor-infiltrating lymphocytes from NSCLC patients (n = 16) treated with anti-PD-1. (A) The Heatmap of gene correlations in CD8+ T cell subsets gives rise to 16 modules or phenotypes. Each color on the left represents the characterization of a different module; (B) Schematic representation of each phenotype based on gene expression; (C) Correlation analysis providing the origin of each module, based on previous CD8+ T cell clustering; (D) Distribution of each phenotype across the UMAP of CD8+ T cells. Median expression is represented by high levels in green/yellow, or low/negative levels in purple. NSCLC: non-small cell lung cancer; UMAP: Uniform Manifold Approximation and Projection.
In a comparison between MPR and non-MPR individuals (Figure 4B,E,H,K,N), module 6 (Figure 4E), a signature of early tumor effector memory cells, was the only one significantly enhanced in patients with complete response. No differences were found between elderly and non-elderly individuals regarding the modules’ scores (Figure 4A,D,G,J,M).
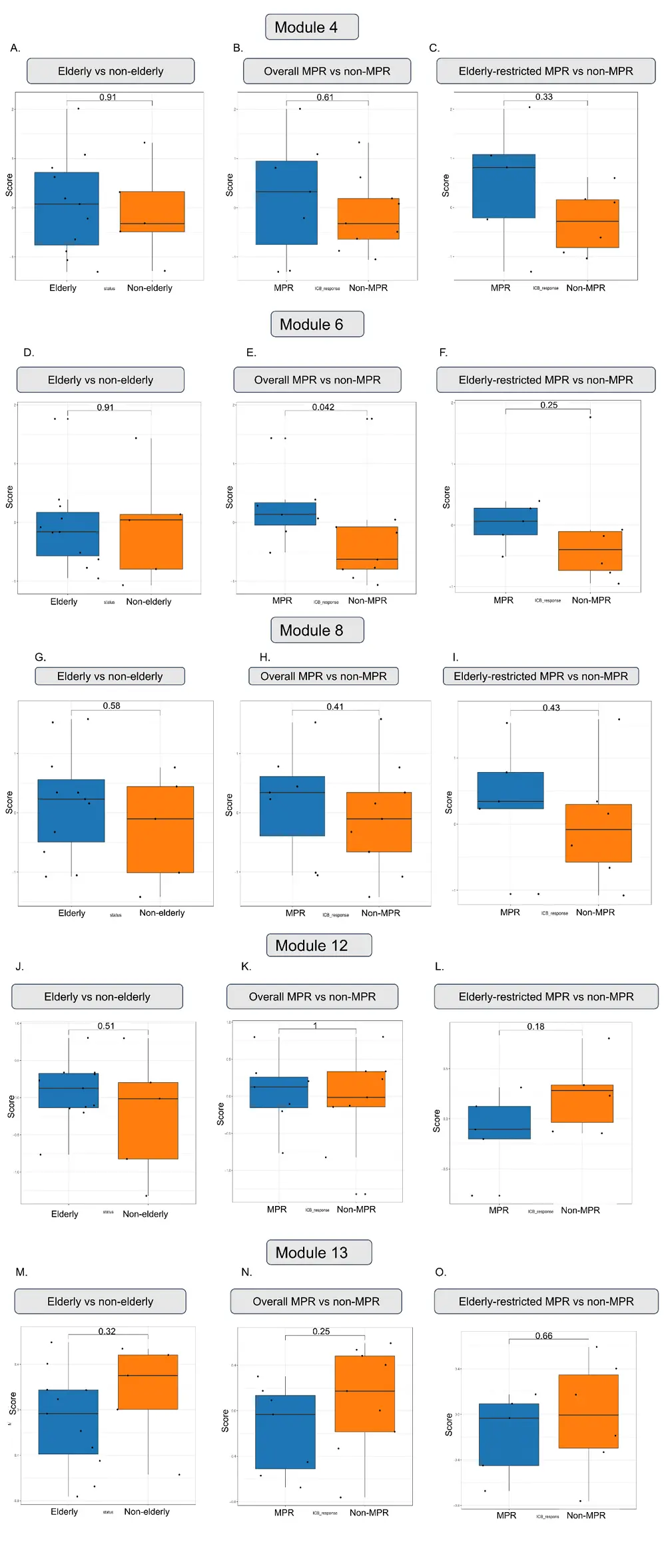
Figure 4. Differences in the upregulation of modules 4, 6, 8, 12 and 13 according to age group (first column: A, D, G, J, M), response to treatment (second column: B, E, H, K, N), and elderly-restricted responsiveness (third column: C, F, I, L, O). Each dot denotes a patient. Bars represent the median with interquartile range. Statistical analysis with non-parametric Wilcoxon test, with P < 0.05 (*), < 0.01 (**), < 0.001 (*).
Therefore, we restricted our analysis only to the elderly and investigated if MPR and non-MPR aged individuals had distinct CD8 molecular programs that could indicate an age-specific profile of response to anti-PD-1. Figure 4C,F,I showed that, despite the absence of significance, aged MPR patients exhibited a median expression score above 0 for modules 4, 6 and 8 compared to the non-MPR (which all scores were negative), suggesting that aged individuals responsive to anti-PD-1 are able to produce a heterogeneous profile of CD8+ responses that are associated with tumor-reactive, memory and effector activity.
3.3 Aged NSCLC patients express higher levels of tumor-reactive T cell genes compared to the non-elderly
Numerous studies focus on the phenotype of tumor-infiltrating lymphocytes as predictive markers for disease resolution[26-28]. A so-called “dysfunctional” phenotype of TILs characterizes tumor-reactive T cells harboring immunosuppressive/exhausted markers, including ENTPD1, HAVCR2, TOX, DUSP4, TIGIT and LAYN, and immune checkpoint inhibitors such as PDCD1 and CTLA-4. Paradoxically, genes representative of activation (LAG3), tissue-residency (ITGAE) and immune recruitment (CXCL13) also compose the signature set of dysfunctional T cells[26-29]. Thus, we integrated this series of genes that coincide with tumor-reactive activity and therapy response and investigated their expression on CD8+ T cells from aged individuals.
The heatmap of tumor-reactive genes presented in Figure 5A shows that DUSP4 was expressed in five out of seven MPR individuals, corroborating the data from Caushi and colleagues[17], who found this gene to be upregulated in responsive individuals. Remarkably, PDCD1 was scarcely expressed in non-MPR patients, whereas its expression in therapy responders showed a high variability. Moreover, seven patients (second to eighth column) presented an absence of expression for at least 10 out of the 11 tumor-reactive genes, five of them (71%) also unresponsive to anti-PD-1. We then crossed these results with the percentage of residual tumor after treatment (Table S3) and observed that these non-MPR patients exhibited a median of 95% (IQR 65-97.5) of residual tumor compared to 67% (IQR 45-93) of the remaining unresponsive patients that expressed tumor-reactive genes, suggesting an important role of dysfunctional-like T cells to tumor clearance.
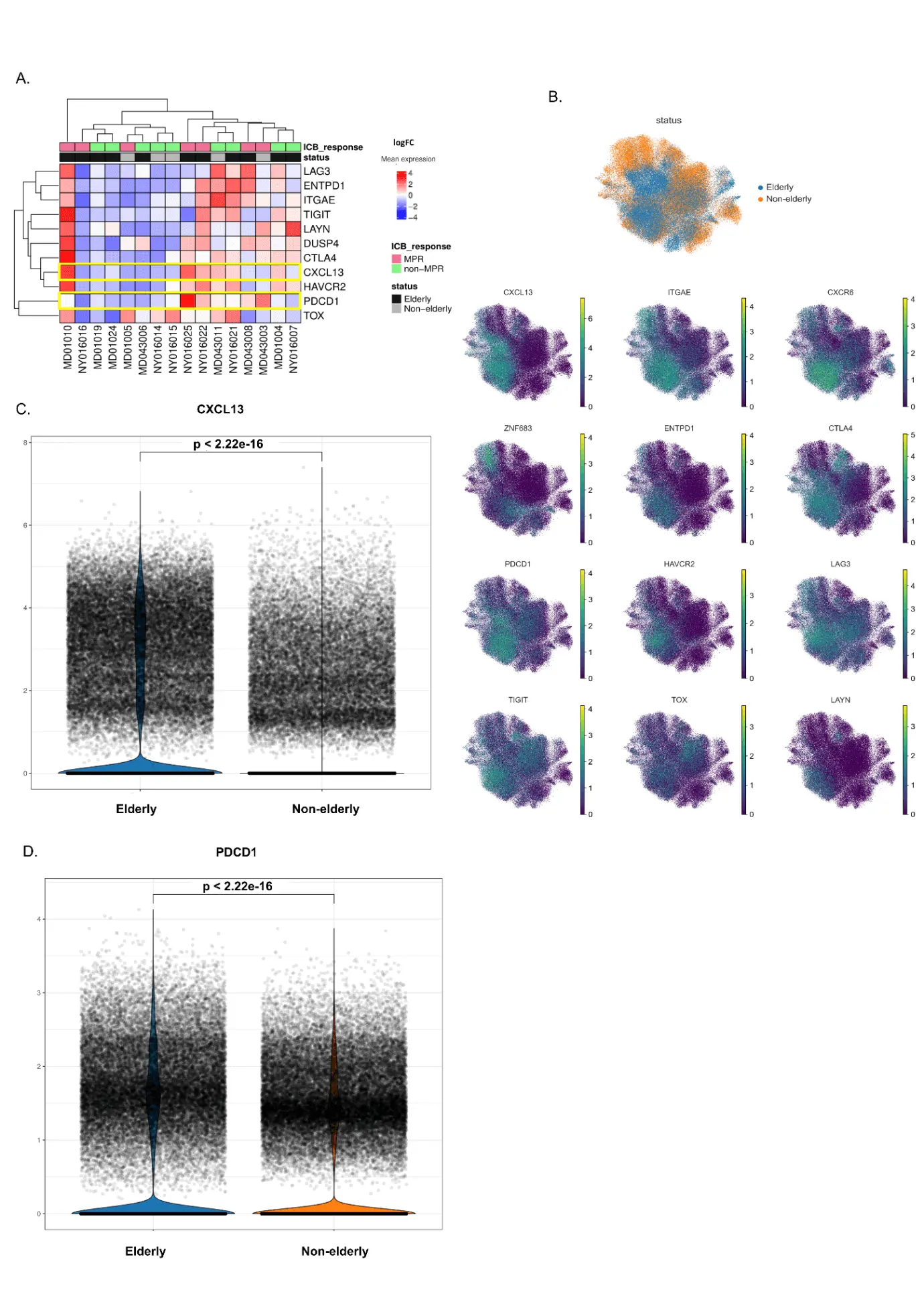
Figure 5. Tumor-reactive genes expressed in elderly and non-elderly NSCLC patients treated with anti-PD-1. (A) Heatmap of tumor-reactive genes' expression according to age group and response to anti-PD-1. Genes designate each row, whereas columns are representative of each patient. Median expression is expressed as high (red) or low/negative (blue); (B) Distribution of tumor-reactive genes across the CD8+ UMAP. Median expression is represented by high levels in green/yellow, or low/negative levels in purple; (C) CXCL13 median expression in elderly (n = 11) and non-elderly (n = 5) NSCLC patients treated with anti-PD-1. The violin distribution designates the density of expression among groups. Statistical analysis with non-parametric Wilcoxon test, with P < 0.05 (*), < 0.01 (**) and < 0.001 (**); (D) PDCD1 median expression in elderly (n = 11) and non-elderly (n = 5) NSCLC patients treated with anti-PD-1. The violin distribution designates the density of expression among groups. Statistical analysis with non-parametric Wilcoxon test, with P < 0.05 (*), < 0.01 (**) and < 0.001 (***). NSCLC: non-small cell lung cancer; MPR: major pathologic response; UMAP: Uniform Manifold Approximation and Projection.
Finally, we performed a comparison analysis of CD8+ tumor-reactive genes between elderly and non-elderly cells, with their respective expressions distributed across the main UMAP (Figure 5B). Strikingly, cells from aged patients exhibited significantly enhanced expression of PDCD1 and CXCL13 genes compared to younger individuals, as presented in Figure 5C,D.
In summary, elderly NSCLC patients treated with anti-PD-1 developed favorable anti-tumoral T cell responses that were enriched with molecular programs and genes associated with tumor-reactivity and therapy efficacy.
4. Discussion
In this work, we reanalyzed the Caushi et al.[17] dataset of tumor-infiltrating lymphocytes and showed that TIL immunosenescence was not restricted to the effects of chronological aging, and that both elderly and younger NSCLC patients were able to develop memory effector CD8+ molecular programs following anti-PD-1 therapy. These results support the dissociation of chronological aging and immunosenescence, especially for the design of cancer immunotherapy studies.
Independent works have suggested different mechanisms by which the elderly could benefit more from immunotherapy than younger subjects. Tumor mutational burden (TMB) is an indicator of neoantigen variability[30] and has thus been associated with ICI response in lung cancer[31,32] and melanoma[33]. In a pan-cancer analysis investigating the relationship between age and biomarkers predictive of ICI efficacy in untreated tumors, Erbe and colleagues[34] found a significant increase in the TMB and in the interferon-gamma signaling of older patients. The expansion of clonal CD8+T cells is a phenomenon often associated with aging and immunosenescence[35]. Interestingly, in a cohort of metastatic melanoma patients (> 70 years) treated with anti-PD-1, Salih et al.[36] observed an elevated TCR clonality in peripheral T cells that positively correlated with therapy response. Additionally, it has been proposed that attenuated FOXP3+ populations in aged (> 60 years), but not younger melanoma patients, are associated with better outcomes in response to anti-PD-1 treatment[11]. A meta-analysis investigating whether age differences could play a role in cancer immunotherapy efficacy found a significantly prolonged overall survival in the elderly (> 65 years) compared to younger individuals in melanoma, NSCLC, renal cell carcinoma, urothelial carcinoma and gastric tumors. Interestingly, the superior benefit was restricted to anti-PD-1 and anti-PD-L1, with no differences regarding anti-CTLA-4 therapy between elderly and non-elderly subjects[9]. Three other studies evaluated age-associated differences in immunotherapy efficacy in melanoma models and found an elevated overall[8,10] and progression-free survival[10] in patients above 65 years old compared to younger individuals, with a particularly higher benefit when the treatment was with anti-PD-1[10,15].
We uncovered an elderly-exclusive enrichment of two gene signatures previously associated with anti-tumor TIL activity. This “dysfunctional-like” T cell phenotype has been shown to associate with response and survival after PD-1[29,37] and PD-L1[38] blockade in lung, advanced basal cell carcinoma and breast cancer patients, respectively. The upregulation of exhaustion markers such as PD-1 (encoded by PDCD1) turns dysfunctional-like CD8+ T cells into a major target for anti-PD-1 therapy. Also, CXCL13 plays a major role in the formation of tertiary lymphoid structures (TLS), which was found to be predictive of response to ICIs and associated with patient survival in different neoplasias[29]. Hence, an elevated expression of PDCD1 and CXCL13 in the elderly could be associated with anti-PD-1 responsiveness and superior immune infiltration through TLS development.
The CD8+ T cells with augmented expression of CXCL13 and PDCD1 following anti-PD-1 therapy could have different origins. There could already be a) more pre-existing TILs in elderly tumors (reinvigoration of dysfunctional-like cells) or b) a higher T cell infiltration from the periphery (activation and expansion of new clones). Either way, it is possible that, as confirmed in many clinical reports, PD-1/PD-L1 blockade works as the most effective pathway to favor tumor clearance in the elderly due to the enriched population of dysfunctional-like T cells’ signatures in this group.
To the best of our knowledge, this is the first study that addresses the molecular profile of tumor-infiltrating lymphocytes in elderly and non-elderly NSCLC patients treated with neoadjuvant anti-PD-1. Further analyses should provide different sources of data-samples before and after treatment, and TCR sequencing—in order to have a refined and precise characterization of T cell responsiveness upon treatment. Samples derived from other compartments (lymph nodes, adjacent normal tissue and peripheral blood) could be useful for tracking T cell dynamics. Moreover, a greater sample size and the inclusion of different cancer models are needed due to the fact that several immunological features are tumor-type specific.
5. Limitations
The data used in this study was derived from CD3-positive cells only, therefore we could not assess the impact of neoadjuvant anti-PD-1 in the TME as a whole. Also, paired samples from patients pre- and post-treatment would give us a better insight of the immunotherapy impact in elderly NSCLC patients. Another important aspect was the variation in cell numbers by sample (ranging from ~1,800 to ~70,000 cells), because some patients had more than one sample collected. For this reason, we have not evaluated cell type composition differences between the elderly and non-elderly patients, since it could affect our model.
6. Conclusion
Elderly NSCLC patients exhibited an enrichment of gene signatures associated to a dysfunctional-like T cell phenotype, characterized by high PDCD1 and CXCL13 expression. Altogether, our findings demonstrate favorable molecular signatures in aged NSCLC individuals following anti-PD-1 treatment and suggest that the recruitment of older adults in immunotherapy clinical trials should not be dismissed solely on the grounds of age.
Supplementary materials
The supplementary material for this article is available at: Supplementary materials.
Acknowledgments
The authors are deeply grateful to Dr. Caushi for his guidance and sincerely appreciate all colleagues in the immunotherapy laboratory for creating an inspiring and exciting work environment.
Authors contribution
Frozza FTB: Conceptualization, formal analysis, investigation, project administration, visualization, writing-original draft, writing-review & editing.
de Mattos P. GFP: Conceptualization, data curation, investigation, methodology, project administration, software, visualization, writing-review & editing.
Bonorino C: Funding acquisition/ Resources, Supervision, writing-review & editing.
Conflicts of interest
Cristina Bonorino is an Editorial Board member of Ageing and Cancer Research & Treatment.The other authors declare no conflicts of interes.
Ethical approval
All ethical disclosures regarding patients’ inclusion and ethics statements (ClinicalTrials.gov ID NCT02259621) can be obtained from the original publication[17] where we stem the data.
Consent to participate
Informed consent was obtained from all patients.
Consent for publication
Not applicable.
Availability of data and materials
The data and materials could be obtained from the corresponding author. All codes reproduced in this analysis can be found on Github (https://github.com/gabriel-pozo/ageing_icb).
Funding information
Fellowships for Fernanda Tereza Bovi Frozza are from CAPES; fellowships for Gabriel F. Pozo de Mattos P. and Cristina Bonorino are from PRONON and CNPq, respectively.
Copyright
© The Author(s) 2023.
References
-
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.[DOI]
-
3. DeSantis CE, Miller KD, Dale W, Mohile SG, Cohen HJ, Leach CR, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69(6):452-467.[DOI]
-
4. Pelissier Vatter FA, Schapiro D, Chang H, Borowsky AD, Lee JK, Parvin B, et al. High-dimensional phenotyping identifies age-emergent cells in human mammary epithelia. Cell Rep. 2018;23(4):1205-1219.[DOI]
-
7. Shah Y, Verma A, Marderstein AR, White J, Bhinder B, Medina JSG, et al. Pan-cancer analysis reveals molecular patterns associated with age. Cell Rep. 2021;37(10):110100.[DOI]
-
12. Yang F, Akinboro O, Lerro C, Rizvi F, Mishra-Kalyani P, Kluetz PG, et al. Abstract A025: Enrollment of older adults in small cell lung cancer (SCLC) clinical trials compared with population-based US incidence estimates. Cancer Res. 2023;83(2 Suppl 1):A025.[DOI]
-
15. Wong SK, Nebhan CA, Johnson DB. Impact of patient age on clinical efficacy and toxicity of checkpoint inhibitor therapy. Front Immunol. 2021;12:786046.[DOI]
-
16. Gomes F, Wong M, Battisti NML, Kordbacheh T, Kiderlen M, Greystoke A, et al. Immunotherapy in older patients with non-small cell lung cancer: Young International Society of Geriatric Oncology position paper. Br J Cancer. 2020;123(6):874-884.[DOI]
-
17. Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EHC, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596(7870):126-132.[DOI]
-
18. Conroy M, Forde PM. Advancing neoadjuvant immunotherapy for lung cancer. Nat Med. 2023;29(3):533-534.[DOI]
-
19. IMMUcan SingleCell RNAseq Database [Internet]. © SIB Swiss Institute of Bioinformatics/Vital-IT 2023. Available from: https://immucanscdb.vital-it.ch/
-
20. TISCH2 [Internet]. Tumor Immune Single-cell Hub 2, TISCH2 project 2022. Available from: http://tisch.comp-genomics.org/
-
21. Gayoso A, Lopez R, Xing G, Boyeau P, Amiri VVP, Hong J, et al. A Python library for probabilistic analysis of single-cell omics data. Nat Biotechnol. 2022;40(2):163-166.[DOI]
-
22. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.[DOI]
-
23. Saul D, Kosinsky RL, Atkinson EJ, Doolittle ML, Zhang X, LeBrasseur NK, et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun. 2022;13(1):4827.[DOI]
-
26. Liu B, Hu X, Feng K, Gao R, Xue Z, Zhang S, et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat Cancer. 2022;3(1):108-121.[DOI]
-
27. Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 2019;176(4):775-789.[DOI]
-
28. Duhen T, Duhen R, Montler R, Moses J, Moudgil T, De Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9(1):2724.[DOI]
-
34. Erbe R, Wang Z, Wu S, Xiu J, Zaidi N, La J, et al. Evaluating the impact of age on immune checkpoint therapy biomarkers. Cell Rep. 2021;36(8):109599.[DOI]
-
35. Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47-56.[DOI]
-
37. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med.2019;25(8): 1251-1259.[DOI]
-
38. Zhang Y, Chen H, Mo H, Hu X, Gao R, Zhao Y, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39(12):1578-1593.[DOI]
Copyright
© The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




