-
Chiral Chemistry (CC, Online ISSN 3106-8405) is a open-access quarterly journal dedicated to publication and dissemination of high-impact, original, and leading research in the field of chiral chemistry and technologies. The journal provides a platform for pioneering theoretical, experimental, and applied studies, promoting fundamental understanding, advancements, and applied innovations across a broad range of scientific and industrial applications. more >
Articles
Enantioselectivity synthesis of isoquinolin-1-one derivatives with C–N axial chirality via cobalt-catalyzed oxidative formal (4+2) cycloaddition: Light or not
-
Developing mild and sustainable strategies for the synthesis of complex molecules is a pivotal yet challenging goal in modern synthesis. While cobalt catalysis offers a sustainable alternative to noble metals, achieving such transformations at room temperature ...
MoreDeveloping mild and sustainable strategies for the synthesis of complex molecules is a pivotal yet challenging goal in modern synthesis. While cobalt catalysis offers a sustainable alternative to noble metals, achieving such transformations at room temperature remains elusive. Herein, we report a cobalt-catalyzed aerobic oxidative asymmetric formal cycloaddition involving C(sp2)–H bond activation of benzamides with unactivated alkynes at room temperature using air as the oxidant. For challenging low-activity substrates, reaction efficiency is enhanced via a photoinduced catalytic cycle. Mechanistic and substrate scope studies indicate that substrate reactivity is influenced by electronic and steric effects.
Less -
Liang-Neng Wang, ... Liang-Qiu Lu
-
DOI: https://doi.org/10.70401/cc.2026.0012 - January 28, 2026
Asymmetric synthesis of mechanically planar chiral rotaxanes via organocatalyzed enantioselective desymmetrization
-
Xiao-Hua Zhou, ... Wei Wang
-
DOI: https://doi.org/10.70401/cc.2026.0011 - January 22, 2026
Development of a scalable iridium-catalyzed asymmetric hydrogenation process for synthesis of chiral 2-methyl-1,2,3,4-tetrahydroquinoline
-
Asymmetric hydrogenation is an efficient tool for rapid synthesis of a diverse range of chiral compounds with high yields and excellent enantioselectivities. Chiral 2-methyl-1,2,3,4-tetrahydro-quinoline is a valuable building block for organic synthesis ...
MoreAsymmetric hydrogenation is an efficient tool for rapid synthesis of a diverse range of chiral compounds with high yields and excellent enantioselectivities. Chiral 2-methyl-1,2,3,4-tetrahydro-quinoline is a valuable building block for organic synthesis and has been widely used in the synthesis of bioactive molecules and chiral ligands. Herein, we report an improved iridium-catalyzed asymmetric hydrogenation of 2-methylquinoline on a hundred-gram scale for efficient synthesis of chiral 2-methyl-1,2,3,4-tetrahydroquinoline with up to 91.4% ee value and an 80,000 turnover number. Optically pure 2-methyl-1,2,3,4-tetrahydroquinoline could be obtained in high yields through recrystallization or chemical resolution with the tartaric acid derivative (L)-DMTA.
Less -
Huan Jing, ... Yong-Gui Zhou
-
DOI: https://doi.org/10.70401/cc.2026.0010 - January 08, 2026
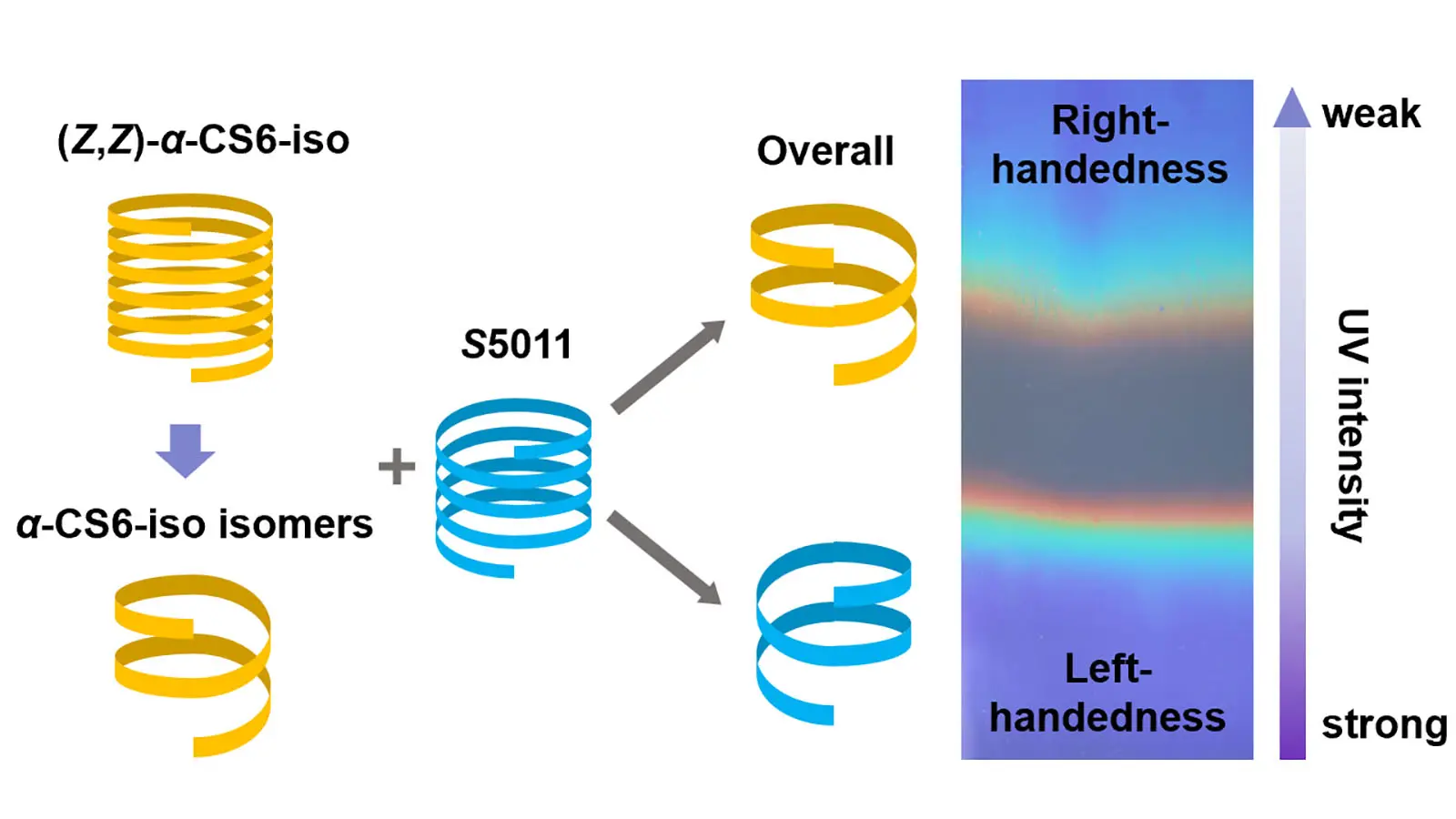
Patterned cholesteric liquid crystal polymer network film with precisely controlled structural colors prepared using a handedness invertible photochromic cholesteric liquid crystal mixture
-
Cholesteric liquid crystal polymer network (CLCN) patterns composed of oppositely handed helical structures are attractive for anti-counterfeiting. Different areas can be observed under the circularly polarized light with opposite handedness. However, ...
MoreCholesteric liquid crystal polymer network (CLCN) patterns composed of oppositely handed helical structures are attractive for anti-counterfeiting. Different areas can be observed under the circularly polarized light with opposite handedness. However, achieving precise control over the reflection band wavelengths of CLCN patterns using photochromic cholesteric liquid crystals (CLCs) remains a significant challenge. Herein, we synthesized a chiral cyanostilbene derivative that demonstrated significant modulation of its helical twisting power upon 365 nm ultraviolet (UV) irradiation. When incorporated into CLC mixtures, this additive enabled precise control over both the handedness and reflection wavelength of the resulting CLCN patterns within seconds under UV exposure. The system allowed for the preparation of colorful CLCN patterns through in-situ photopolymerization. These findings demonstrate that the developed CLC mixtures are well-suited for high-throughput fabrication of CLCN patterns on industrial coating lines.
Less -
Xingchen Liu, ... Yonggang Yang
-
DOI: https://doi.org/10.70401/cc.2025.0009 - December 31, 2025
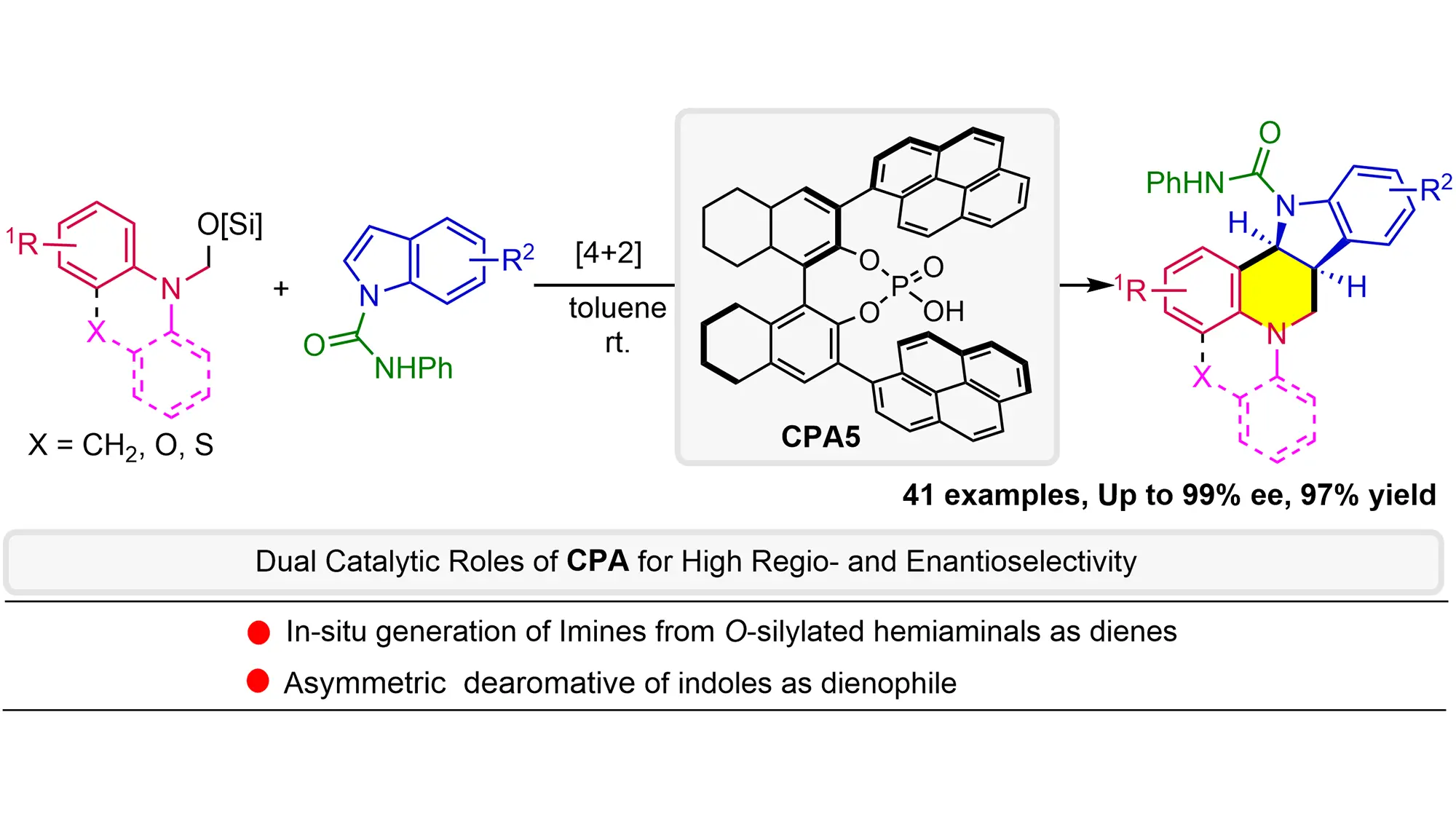
Catalytic asymmetric dearomative formal [4+2] annulation of indoles with O-Silylated hemiaminals as dienes: The dual role of chiral phosphoric acid
-
O-Silylated hemiaminals are utilized as elegant imine precursors in the formal asymmetric [4+2] annulation of indoles for the first time, wherein chiral phosphoric acid (CPA) acts (1) as a Brønsted acid catalyst to facilitate methanimine formation ...
MoreO-Silylated hemiaminals are utilized as elegant imine precursors in the formal asymmetric [4+2] annulation of indoles for the first time, wherein chiral phosphoric acid (CPA) acts (1) as a Brønsted acid catalyst to facilitate methanimine formation under mild conditions and then (2) as an anion-binding catalyst for dearomative annulation. This methodology exhibits a broad substrate scope with remarkable functional group tolerance and enantioselectivity (up to 97% yield and 99% ee), providing straightforward access to the challenging indoline-fused tetrahydroquinolines bearing multiple stereogenic centers. Mechanistic studies reveal the critical role of PhNHCO- groups in enhancing both reactivity and enantioselectivity, probably due to non-covalent interactions with CPA. The kinetic isotope effects experiment and negative linear Hammett correlation suggest a concerted process.
Less -
Nan-Fang Mo, ... Zheng-Hui Guan
-
DOI: https://doi.org/10.70401/cc.2025.0008 - December 26, 2025
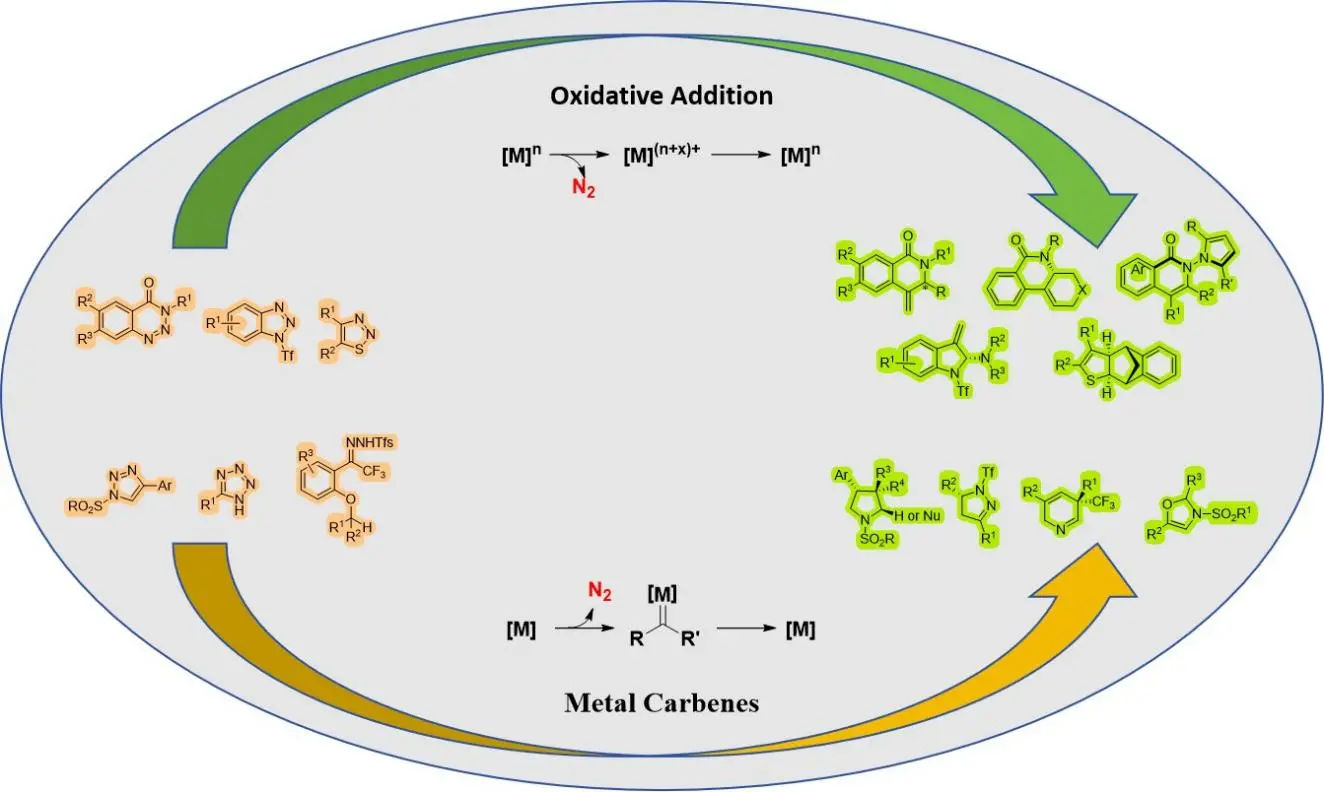
Transition-metal-catalyzed asymmetric denitrogenative transannulation
-
Over the past few decades, denitrogenation has proven to be an effective method for synthesizing high-value chiral heterocyclic compounds. These compounds find widespread applications in pharmaceutical chemistry, drug development, and natural product ...
MoreOver the past few decades, denitrogenation has proven to be an effective method for synthesizing high-value chiral heterocyclic compounds. These compounds find widespread applications in pharmaceutical chemistry, drug development, and natural product synthesis. Denitrogenation demonstrates high activity and can engage in cyclization reactions with olefins, alkynes, carbon-heterocycles, aldehydes, and other reagents. This one-step operation enables the rapid construction of chiral heterocycles such as pyrroles and indoles, significantly shortening complex synthetic pathways. Innovations in chiral ligands, optimization of catalytic systems, and detailed studies on mechanisms have significantly enhanced the enantioselectivity and substrate applicability of denitrogenation reactions. This review highlights recent advancements in the synthesis of chiral heterocycles via denitrogenation reactions and systematically examines the reaction characteristics of various metal catalytic systems.
Less -
Wen-Ge Guo, Ren-Rong Liu
-
DOI: https://doi.org/10.70401/cc.2025.0001 - November 06, 2025
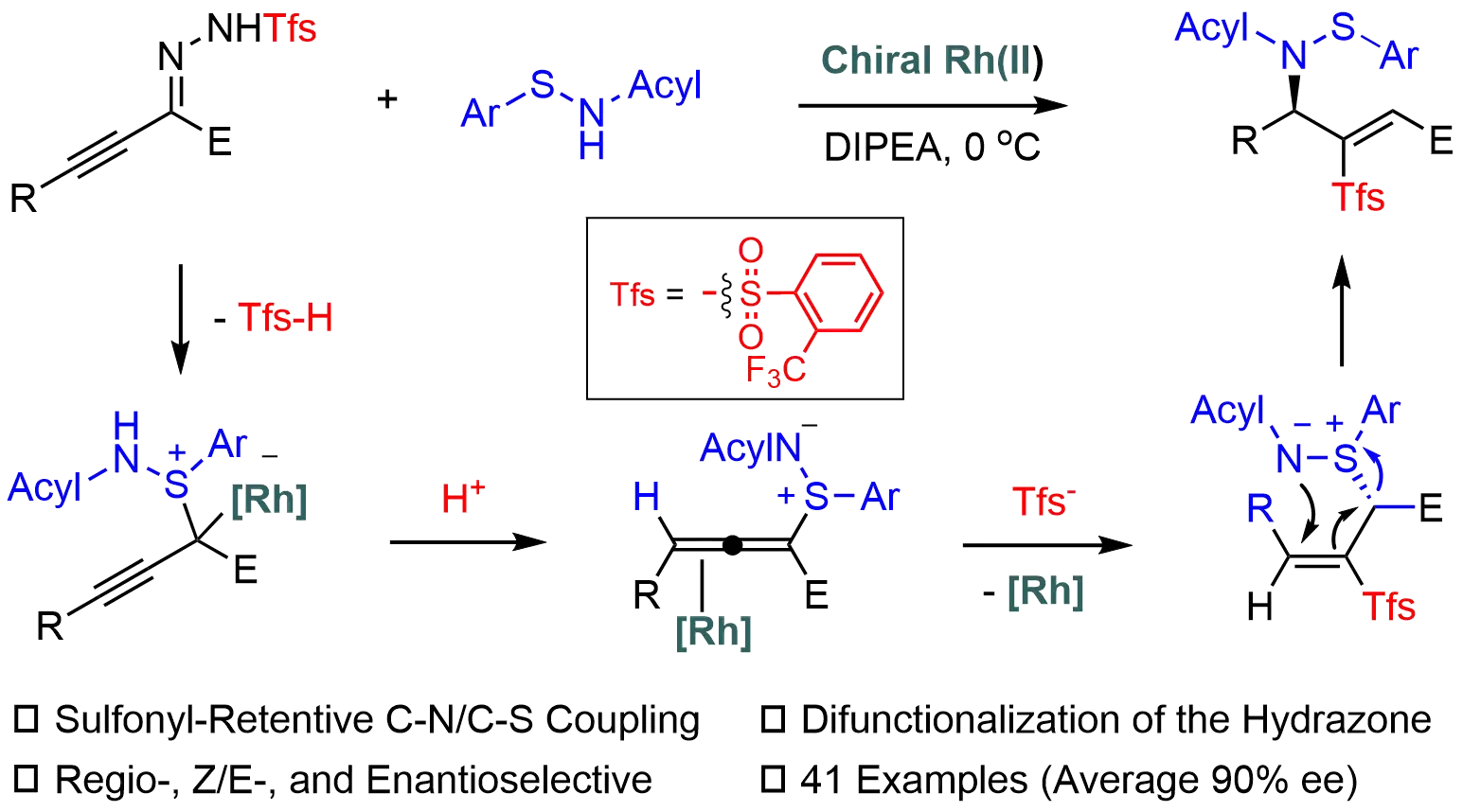
Enantioselective aza-Mislow-Evans rearrangement through S-allenylation of sulfenamides with alkynyl carbenes
-
Sulfonylhydrazones are valuable carbene precursors in asymmetric synthesis; however, their use typically generates sulfinic acid, which is inevitably discarded as stoichiometric waste. In this work, the carbene chemistry and sulfur chemistry are integrated ...
MoreSulfonylhydrazones are valuable carbene precursors in asymmetric synthesis; however, their use typically generates sulfinic acid, which is inevitably discarded as stoichiometric waste. In this work, the carbene chemistry and sulfur chemistry are integrated in Rh-catalyzed asymmetric sulfonyl retentive S,N-difunctionalization of alkynyl N-sulfonylhydrazones with sulfenamides. The sulfonyl group or the corresponding sulfinic acid is retained in a formal migration. This mild and efficient protocol allows for straightforward construction of enantioenriched sulfonyl-based allylic sulfenamides with excellent chemo-, E/Z-, and enantioselectivity, thereby offering a creative strategy for achieving more atom-economic transformations of carbene precursors. Mechanistic studies reveal a three-step process involving S-allenylation, hydrosulfonylation, and an aza-Mislow-Evans rearrangement, with the hydrosulfonylation step governing enantioselectivity.
Less -
Bin Wei, ... Xingwei Li
-
DOI: https://doi.org/10.70401/cc.2025.0004 - December 12, 2025
Advances in catalytic asymmetric hydrogenation of third-row heteroatom-substituted alkenes
-
The asymmetric hydrogenation of vinyl silanes, vinyl sulfides, vinyl phosphines, and vinyl chlorides, those substituted with heteroatoms from the third-row of the periodic table, has emerged as a valuable and environmentally friendly method for the construction ...
MoreThe asymmetric hydrogenation of vinyl silanes, vinyl sulfides, vinyl phosphines, and vinyl chlorides, those substituted with heteroatoms from the third-row of the periodic table, has emerged as a valuable and environmentally friendly method for the construction of the related optically active organosilanes, organosulfides, organophosphine, and organochlorides. These compounds have shown considerable potential for preparing functional molecules and synthesizing natural products. Over the past few decades, considerable research efforts have focused on the design and development of transition-metal catalysts featuring chiral ligands for the asymmetric hydrogenation of such substrates. In parallel, in-depth mechanistic studies have been conducted to elucidate the pathways of these enantioselective hydrogenation reactions, significantly advancing the understanding of their catalytic behavior and stereocontrol. This review focuses on the recent momentum and key advancements in the enantioselective hydrogenation of vinyl silanes, vinyl sulfides, and vinyl chlorides. In addition, given the widespread industrial interest in these compounds, the practical utility of this transformation in the synthesis of chiral silanes, chiral thioethers, chiral alkyl chlorides, as well as related derivatives, is also discussed.
Less -
Jian Zhang, ... Wanbin Zhang
-
DOI: https://doi.org/10.70401/cc.2025.0002 - November 27, 2025
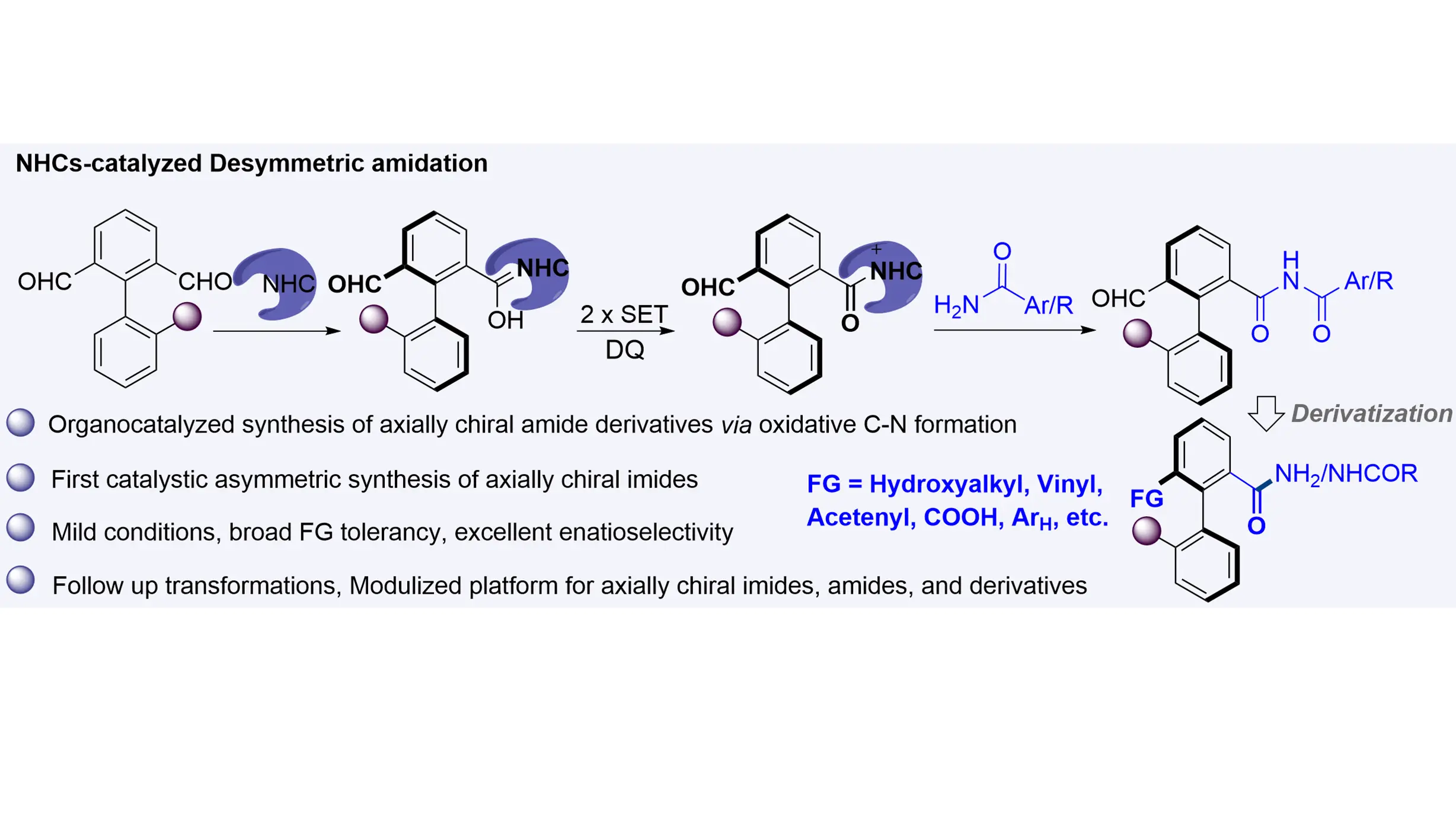
NHCs-catalyzed enantioselective synthesis of biaryl axially chiral imides
-
The synthesis of biaryl axially chiral amides and their derivatives—compounds that have shown promise as additives or catalysts in asymmetric catalysis—has traditionally relied on transition-metal catalysts. Herein, we report an NHC-catalyzed organocatalytic ...
MoreThe synthesis of biaryl axially chiral amides and their derivatives—compounds that have shown promise as additives or catalysts in asymmetric catalysis—has traditionally relied on transition-metal catalysts. Herein, we report an NHC-catalyzed organocatalytic atropoenantioselective amidation between axially prochiral biaryl dialdehydes and amides that efficiently affords axially chiral imides. This method operates under metal-free and mild conditions, exhibits broad functional group tolerance and substrate scope, and delivers products with excellent enantioselectivities. Furthermore, a wide variety of axially chiral imides, amides, and related derivatives can be accessed through enantio-retentive transformations, offering a versatile and attractive strategy for their synthesis.
Less -
Yingtao Wu, ... Qian Zhang
-
DOI: https://doi.org/10.70401/cc.2025.0005 - December 15, 2025
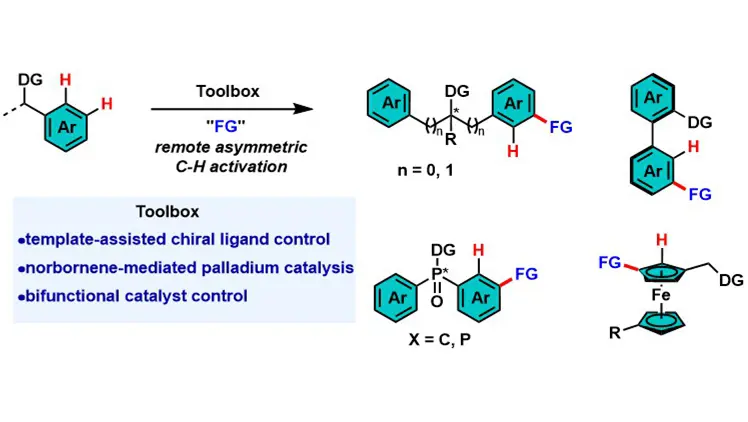
Transition metal-catalyzed remote asymmetric C–H activation of arenes
-
Transition metal-catalyzed asymmetric C–H activation is vital for chiral molecule synthesis but faces challenges in remote C–H functionalization due to traditional metallacycle constraints and difficulties in long-range chiral recognition. This review ...
MoreTransition metal-catalyzed asymmetric C–H activation is vital for chiral molecule synthesis but faces challenges in remote C–H functionalization due to traditional metallacycle constraints and difficulties in long-range chiral recognition. This review summarizes three core strategies to address these issues: template-assisted chiral ligand control, norbornene-mediated palladium catalysis, and bifunctional catalyst control. These strategies achieve high enantioselectivity for diverse chiral architectures. Future directions include expanding to para-C–H bonds of arenes and aliphatic C–H bonds, developing robust chiral mediators/ligands, and applying the methodology to natural products and complex materials.
Less -
Lili Chen, Senmiao Xu
-
DOI: https://doi.org/10.70401/cc.2025.0006 - December 16, 2025
Transition-metal-catalyzed asymmetric denitrogenative transannulation
-
Over the past few decades, denitrogenation has proven to be an effective method for synthesizing high-value chiral heterocyclic compounds. These compounds find widespread applications in pharmaceutical chemistry, drug development, and natural product ...
MoreOver the past few decades, denitrogenation has proven to be an effective method for synthesizing high-value chiral heterocyclic compounds. These compounds find widespread applications in pharmaceutical chemistry, drug development, and natural product synthesis. Denitrogenation demonstrates high activity and can engage in cyclization reactions with olefins, alkynes, carbon-heterocycles, aldehydes, and other reagents. This one-step operation enables the rapid construction of chiral heterocycles such as pyrroles and indoles, significantly shortening complex synthetic pathways. Innovations in chiral ligands, optimization of catalytic systems, and detailed studies on mechanisms have significantly enhanced the enantioselectivity and substrate applicability of denitrogenation reactions. This review highlights recent advancements in the synthesis of chiral heterocycles via denitrogenation reactions and systematically examines the reaction characteristics of various metal catalytic systems.
Less -
Wen-Ge Guo, Ren-Rong Liu
-
DOI: https://doi.org/10.70401/cc.2025.0001 - November 06, 2025
Enantioselective aza-Mislow-Evans rearrangement through S-allenylation of sulfenamides with alkynyl carbenes
-
Sulfonylhydrazones are valuable carbene precursors in asymmetric synthesis; however, their use typically generates sulfinic acid, which is inevitably discarded as stoichiometric waste. In this work, the carbene chemistry and sulfur chemistry are integrated ...
MoreSulfonylhydrazones are valuable carbene precursors in asymmetric synthesis; however, their use typically generates sulfinic acid, which is inevitably discarded as stoichiometric waste. In this work, the carbene chemistry and sulfur chemistry are integrated in Rh-catalyzed asymmetric sulfonyl retentive S,N-difunctionalization of alkynyl N-sulfonylhydrazones with sulfenamides. The sulfonyl group or the corresponding sulfinic acid is retained in a formal migration. This mild and efficient protocol allows for straightforward construction of enantioenriched sulfonyl-based allylic sulfenamides with excellent chemo-, E/Z-, and enantioselectivity, thereby offering a creative strategy for achieving more atom-economic transformations of carbene precursors. Mechanistic studies reveal a three-step process involving S-allenylation, hydrosulfonylation, and an aza-Mislow-Evans rearrangement, with the hydrosulfonylation step governing enantioselectivity.
Less -
Bin Wei, ... Xingwei Li
-
DOI: https://doi.org/10.70401/cc.2025.0004 - December 12, 2025
Advances in catalytic asymmetric hydrogenation of third-row heteroatom-substituted alkenes
-
The asymmetric hydrogenation of vinyl silanes, vinyl sulfides, vinyl phosphines, and vinyl chlorides, those substituted with heteroatoms from the third-row of the periodic table, has emerged as a valuable and environmentally friendly method for the construction ...
MoreThe asymmetric hydrogenation of vinyl silanes, vinyl sulfides, vinyl phosphines, and vinyl chlorides, those substituted with heteroatoms from the third-row of the periodic table, has emerged as a valuable and environmentally friendly method for the construction of the related optically active organosilanes, organosulfides, organophosphine, and organochlorides. These compounds have shown considerable potential for preparing functional molecules and synthesizing natural products. Over the past few decades, considerable research efforts have focused on the design and development of transition-metal catalysts featuring chiral ligands for the asymmetric hydrogenation of such substrates. In parallel, in-depth mechanistic studies have been conducted to elucidate the pathways of these enantioselective hydrogenation reactions, significantly advancing the understanding of their catalytic behavior and stereocontrol. This review focuses on the recent momentum and key advancements in the enantioselective hydrogenation of vinyl silanes, vinyl sulfides, and vinyl chlorides. In addition, given the widespread industrial interest in these compounds, the practical utility of this transformation in the synthesis of chiral silanes, chiral thioethers, chiral alkyl chlorides, as well as related derivatives, is also discussed.
Less -
Jian Zhang, ... Wanbin Zhang
-
DOI: https://doi.org/10.70401/cc.2025.0002 - November 27, 2025
NHCs-catalyzed enantioselective synthesis of biaryl axially chiral imides
-
The synthesis of biaryl axially chiral amides and their derivatives—compounds that have shown promise as additives or catalysts in asymmetric catalysis—has traditionally relied on transition-metal catalysts. Herein, we report an NHC-catalyzed organocatalytic ...
MoreThe synthesis of biaryl axially chiral amides and their derivatives—compounds that have shown promise as additives or catalysts in asymmetric catalysis—has traditionally relied on transition-metal catalysts. Herein, we report an NHC-catalyzed organocatalytic atropoenantioselective amidation between axially prochiral biaryl dialdehydes and amides that efficiently affords axially chiral imides. This method operates under metal-free and mild conditions, exhibits broad functional group tolerance and substrate scope, and delivers products with excellent enantioselectivities. Furthermore, a wide variety of axially chiral imides, amides, and related derivatives can be accessed through enantio-retentive transformations, offering a versatile and attractive strategy for their synthesis.
Less -
Yingtao Wu, ... Qian Zhang
-
DOI: https://doi.org/10.70401/cc.2025.0005 - December 15, 2025
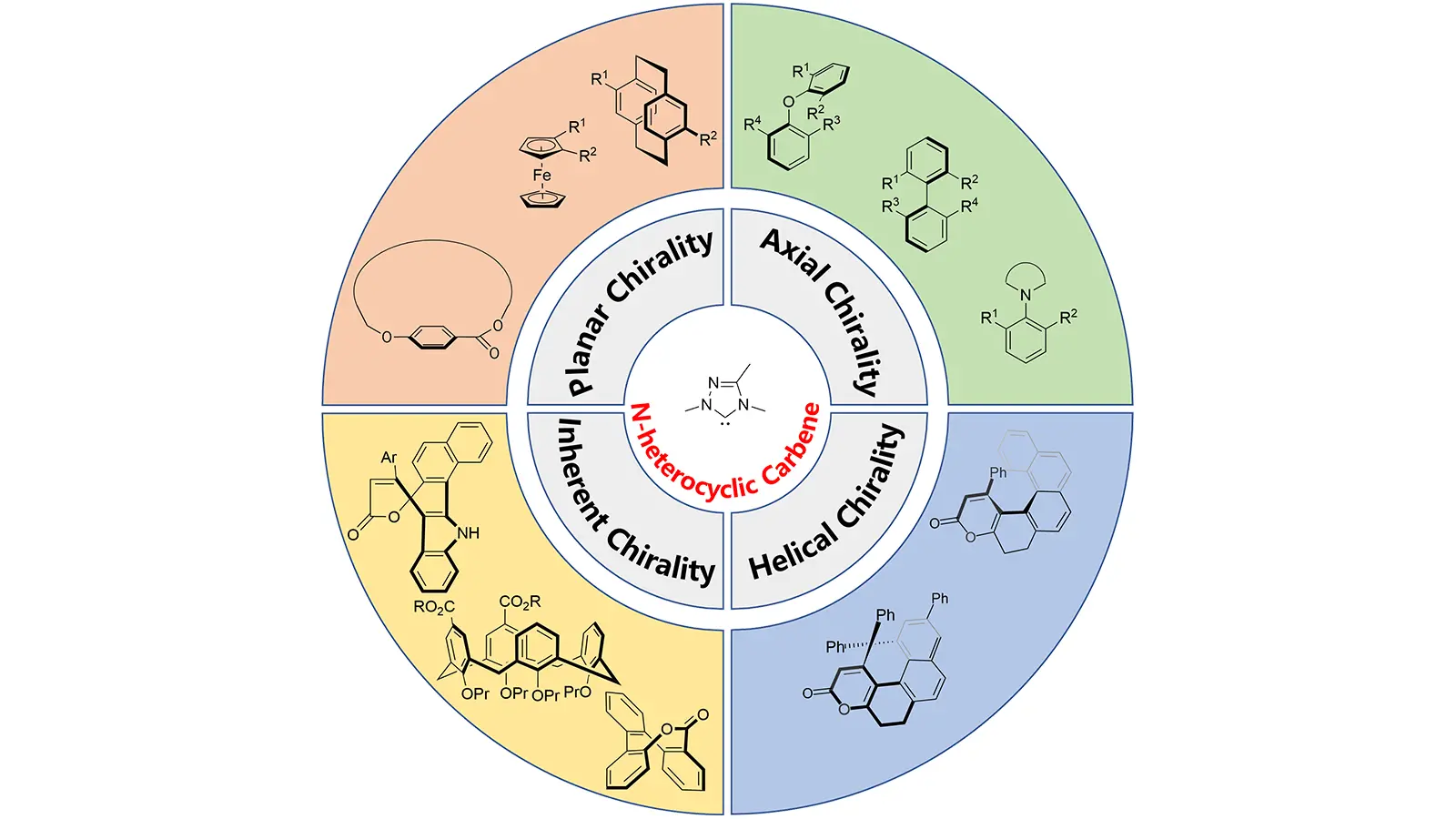
Asymmetric construction of non-central chiral compounds with N-heterocyclic carbenes organocatalysis
-
This review systematically summarizes recent advances in N-heterocyclic carbene (NHC)-catalyzed asymmetric synthesis of non-central chiral compounds. The key synthetic strategies include oxidative and redox-neutral acylation reactions, LUMO-activation-mediated ...
MoreThis review systematically summarizes recent advances in N-heterocyclic carbene (NHC)-catalyzed asymmetric synthesis of non-central chiral compounds. The key synthetic strategies include oxidative and redox-neutral acylation reactions, LUMO-activation-mediated conjugate additions, cyclization reactions, benzoin/Stetter reactions, and imine activation processes. In this work, the versatility of NHCs in asymmetric synthesis is highlighted by their capacity to achieve high stereoselectivity across a wide range of substrates. These advances provide valuable insights for applications in chiral drug development, materials science, and catalysis.
Less -
Peng Chen, ... Zhichao Jin
-
DOI: https://doi.org/10.70401/cc.2025.0003 - December 09, 2025