Wei Wang, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, China. E-mail: wwang@chem.ecnu.edu.cn
Graphical Abstract
1. Introduction
Rotaxanes are a fundamental class of mechanically interlocked molecules, in which a macrocyclic component is threaded onto a dumbbell-shaped axle component[1]. Notably, even in the absence of classical chiral elements within the subcomponents, rotaxanes can exhibit unique mechanically planar chirality (MPC), which originates from the spatial arrangement of the subcomponents connected through mechanical bonds[2]. Taking a [2]rotaxane as an example, chirality can emerge upon the formation of a mechanical bond when a Cnh symmetric macrocyclic component encircles a Cnv symmetric axle component (Figure 1a). As early as 1971, Schill introduced the concept of “cyclochirality” to describe asymmetry within rotaxane molecules[3]. It was until 1997 that Vögtle and Okamoto et al. successfully resolved the enantiomers of rotaxanes using chiral stationary phase high-performance liquid chromatography (CSP-HPLC) technology, confirming the existence of this type of chirality and achieving the successful preparation of enantiomerically pure MPC rotaxanes[4]. Subsequently, Takata et al. suggested that this form of chirality in rotaxanes is analogous to “planar chirality” in covalent structures[5]. More recently, Goldup et al. extended the nomenclature by proposing that such rotaxanes should be recognized as “mechanically planar chiral” to emphasize the role of the mechanical bond in establishing their stereostructure[2].
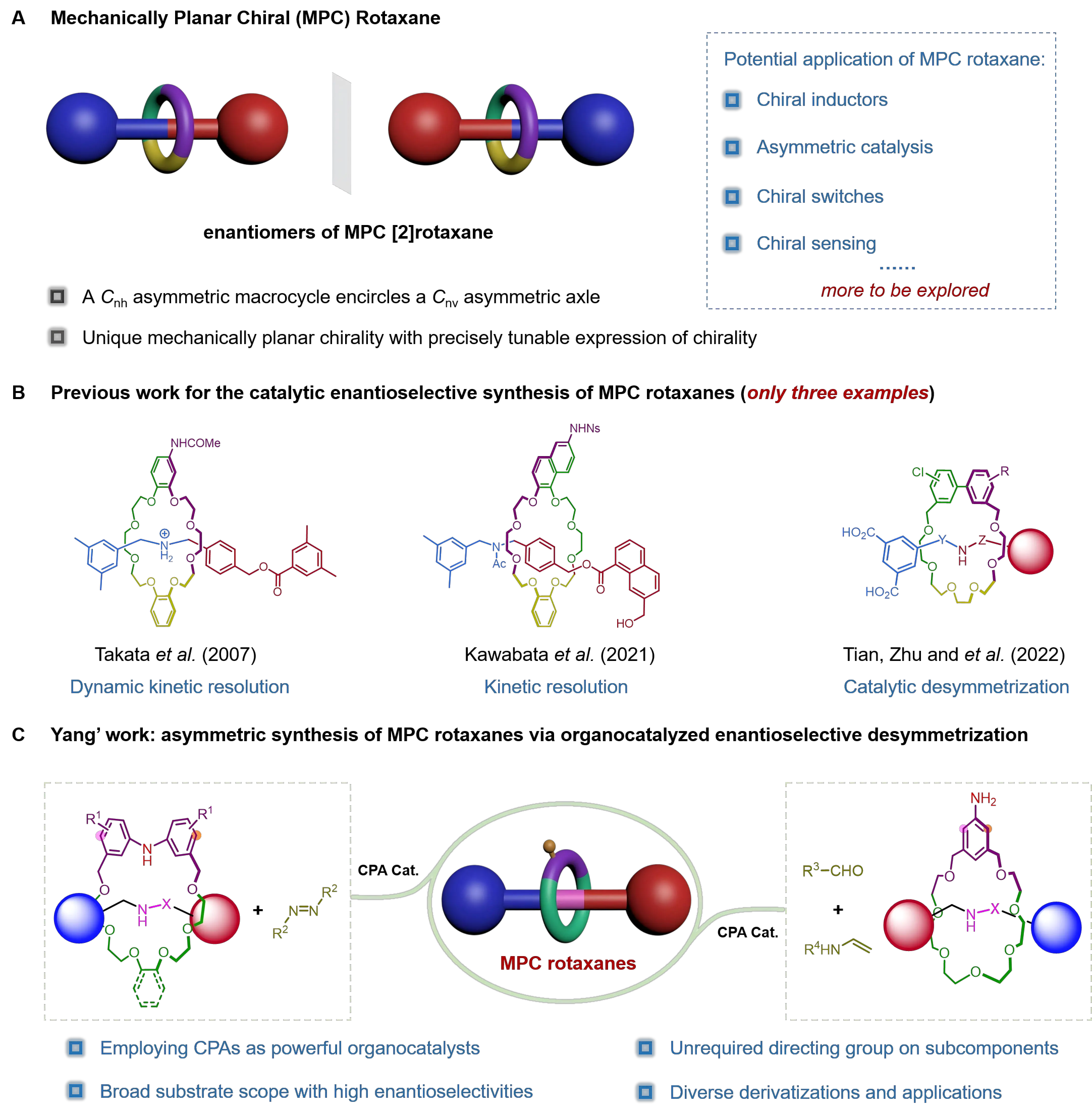
Figure 1. Asymmetric synthesis of mechanically planar chiral rotaxanes. (A) The model of the MPC [2]rotaxanes and their applications; (B) Previous approaches for the catalytic asymmetric synthesis of MPC rotaxanes; (C) Organocatalyzed enantioselective desymmetrization strategy developed by Yang et al. for the asymmetric synthesis of MPC rotaxanes[13,14]. MPC: mechanically planar chirality.
Compared with traditional chiral rotaxanes, mechanically planar chirality adds a new dimension to the chirality of rotaxanes, and the unique spatial structures and controllable motion characteristics of rotaxanes further enrich their chirality connotation, particularly in terms of their precise and tunable chirality expression, endowing them with significant application potentials in fields such as chiral recognition[6], asymmetric catalysis[7], and chiroptical switches[8]. Despite their promising prospects, the synthesis of MPC rotaxanes still largely relies on CSP-HPLC for enantiomer separation. In recent years, the Goldup group has developed practical synthetic routes to MPC rotaxanes by employing chiral derivatization[9] and chiral auxiliary strategies[7]. Additionally, the Leigh group has reported a one-step metal-free active template method using a chiral auxiliary as a leaving group to directly construct MPC rotaxanes, albeit with only moderate enantioselectivity[10]. Asymmetric catalysis represents an ideal strategy for the efficient synthesis of MPC rotaxanes, offering notable advantages such as high stereoselectivity and atom economy. In 2007, the Takata group reported the first asymmetric catalytic synthesis of MPC rotaxanes through dynamic kinetic resolution by catalytic asymmetric acylation of a pseudorotaxane, although the product was obtained with only 4% e.e.[5]. In 2021, the Kawabata group developed a kinetic resolution strategy that afforded MPC rotaxanes with up to 99.9% e.e. through asymmetric acylation of a racemic rotaxane precursor. However, the substrate scope of this method remains to be explored[11]. In 2022, Zhu, Tian, and co-workers employed a palladium-catalyzed Suzuki-Miyaura reaction to access enantiomerically enriched MPC rotaxanes through desymmetrizing arylation of a prochiral rotaxane precursor (Figure 1b)[12]. It is worth noting that these current reports emphasize the essential role of the interactions between directing groups in the non-reactive subunits of the rotaxanes and the catalysts for achieving efficient stereocontrol. However, the requirement to install directing groups on rotaxane substrates not only inevitably increases synthetic complexity but also constrains subsequent diversification and application-oriented investigations.
This highlight summarizes and discusses the recent advances in the catalytic asymmetric synthesis of MPC rotaxanes. These approaches employed chiral phosphoric acids (CPAs) as organocatalysts for the asymmetric electrophilic aromatic amination and the Povarov reaction, affording the corresponding MPC rotaxanes with high stereoselectivity through a desymmetrization strategy
2. Organocatalyzed Enantioselective Desymmetrization for the Asymmetric Synthesis of MPC Rotaxanes.
A recent study by Yang and co-workers constitutes significant progress in the field of asymmetric catalytic synthesis of MPC rotaxanes. Their approach involved a CPA-catalyzed enantioselective electrophilic aromatic amination reaction of prochiral rotaxanes with azodicarboxylates[13]. In the prochiral rotaxanes, which feature a Cnv symmetric axle component alongside a rotationally symmetric diarylamine-containing macrocyclic component, a single aromatic amination on the macrocycle will break the molecular symmetry, resulting in the emergence of mechanically planar chirality. By employing 10 mol% of a CPA catalyst in chloroform at room temperature, a series of MPC rotaxanes were obtained with high enantioselectivities. Investigation of the substrate scope revealed that diverse modifications on the axle or macrocycle component of the prochiral rotaxanes were well tolerated, affording the corresponding MPC rotaxanes with high e.e. values. Interestingly, introducing alkyl or aryl substituents at the meta-position of the diarylamine moiety on the macrocycle yields chiral rotaxanes that simultaneously possess both mechanically planar chirality and C–N axial chirality. Subsequent direct hydrogenation of these products efficiently removes the additional C–N axial chirality, providing convenient access to the desired MPC rotaxanes.
Mechanistic studies revealed that the stereoselectivity of this reaction originates from a sequential desymmetrization and kinetic resolution process. The enantioselective desymmetrization step favored the formation of the Rmp configuration product as the major enantiomer, and the subsequent kinetic resolution step favored the rapid consumption of the minor enantiomer, thus collectively enhancing the enantioselectivity of the MPC rotaxane product. Based on control studies involving single-crystal structural analysis of the prochiral rotaxane, a plausible reaction mechanism was proposed. The axle and macrocycle components of the rotaxane are preorganized through hydrogen bonding, π–π interactions, and C–H···π contacts, which partially restrict their conformational flexibility. Subsequently, both the diarylamine N–H group and the azodicarboxylate are activated by the CPA catalyst through a dual hydrogen-bonding interaction, which facilitates the selective addition of aniline A’ at the para-position of the azodicarboxylate, thereby enabling an effective stereocontrol. It is noteworthy that the mechanism involving the B-ring engaging in a parallelly displaced π–π interaction with the axle, while the less sterically hindered A-ring attacks the azodicarboxylate, is also plausible
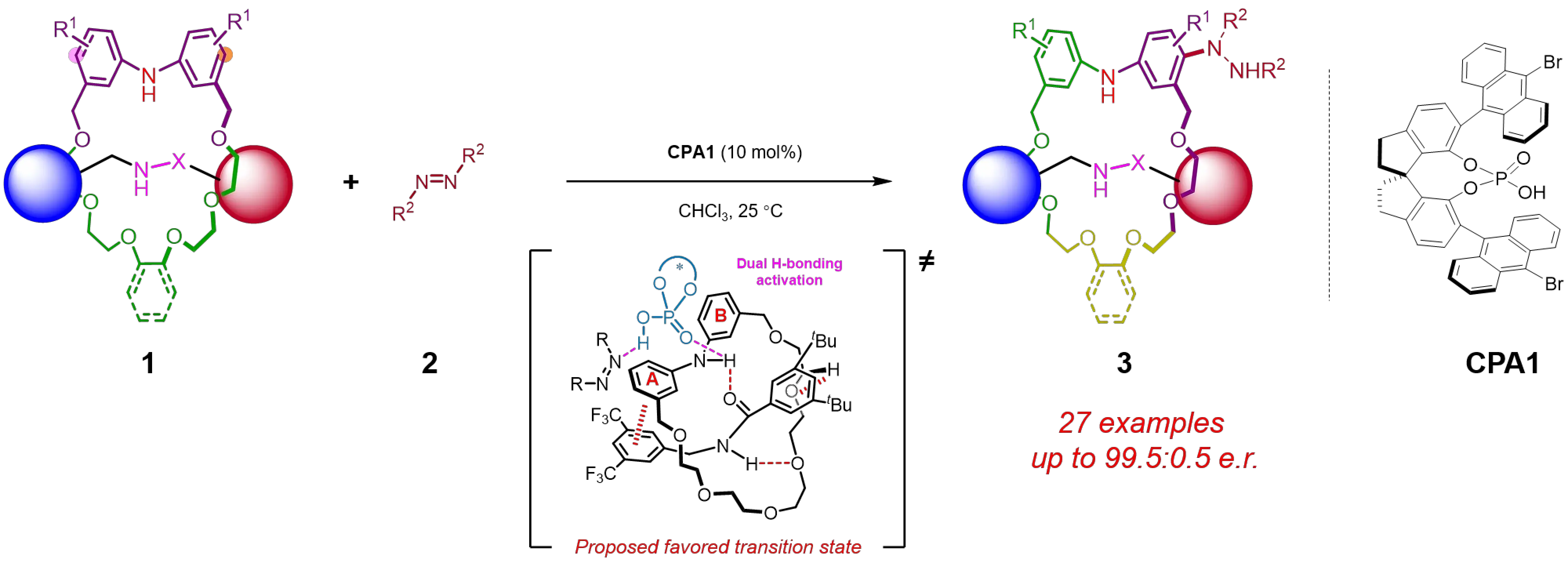
Figure 2. Enantioselective synthesis of MPC rotaxane via CPA-catalyzed electrophilic aromatic amination reaction. MPC: mechanically planar chirality; CPA: chiral phosphoric acid.
Very recently, the same group has developed a CPA-catalyzed asymmetric Povarov reaction as another elegant methodology for the efficient enantioselective synthesis of MPC rotaxanes[14]. In this work, a variety of chiral rotaxanes were obtained in good yields with high diastereoselectivities by employing 10 mol% CPA catalyst to catalyze the three-component Povarov reaction involving a prochiral rotaxane, benzaldehyde, and enamide in ether at -10 °C. Subsequent aromatization of the tetrahydroquinoline (THQ) moiety in one-pot would eliminated the central chirality, ultimately affording the target products in which mechanically planar chirality constitutes the sole stereogenic element. Stereocontrol was implemented through a desymmetrization strategy, in which the chiral catalyst enabled precise regioselective cyclization at one ortho-position of the aniline moiety, thereby imparting directionality to the macrocycle and breaking the symmetry of the prochiral rotaxane. Notably, this methodology exhibited a broad substrate scope and good functional group compatibility. The catalytic transformation proceeded smoothly with a variety of aryl and alkyl aldehydes, affording the products with high enantioselectivities, and diverse prochiral rotaxane substrates were also compatible. Interestingly, by adopting a stepwise approach, diastereomers of the rotaxanes bearing a central chiral unit were first isolated, and then oxidized and aromatized by DDQ to remove the central chirality, affording both enantiomers of the MPC rotaxanes with high e.e. values.
To gain a better understanding of the origin of the enantioselectivity, a plausible reaction mechanism was proposed. The reaction commenced with the condensation of the aniline-containing prochiral rotaxane with the aldehyde to yield the imine intermediate, which adopts a favored mechanical geometry conducive to stereocontrol. Subsequently, both the imine intermediate and the enamide are activated by the CPA catalyst through a dual hydrogen-bonding network, leading to the selective formation of a
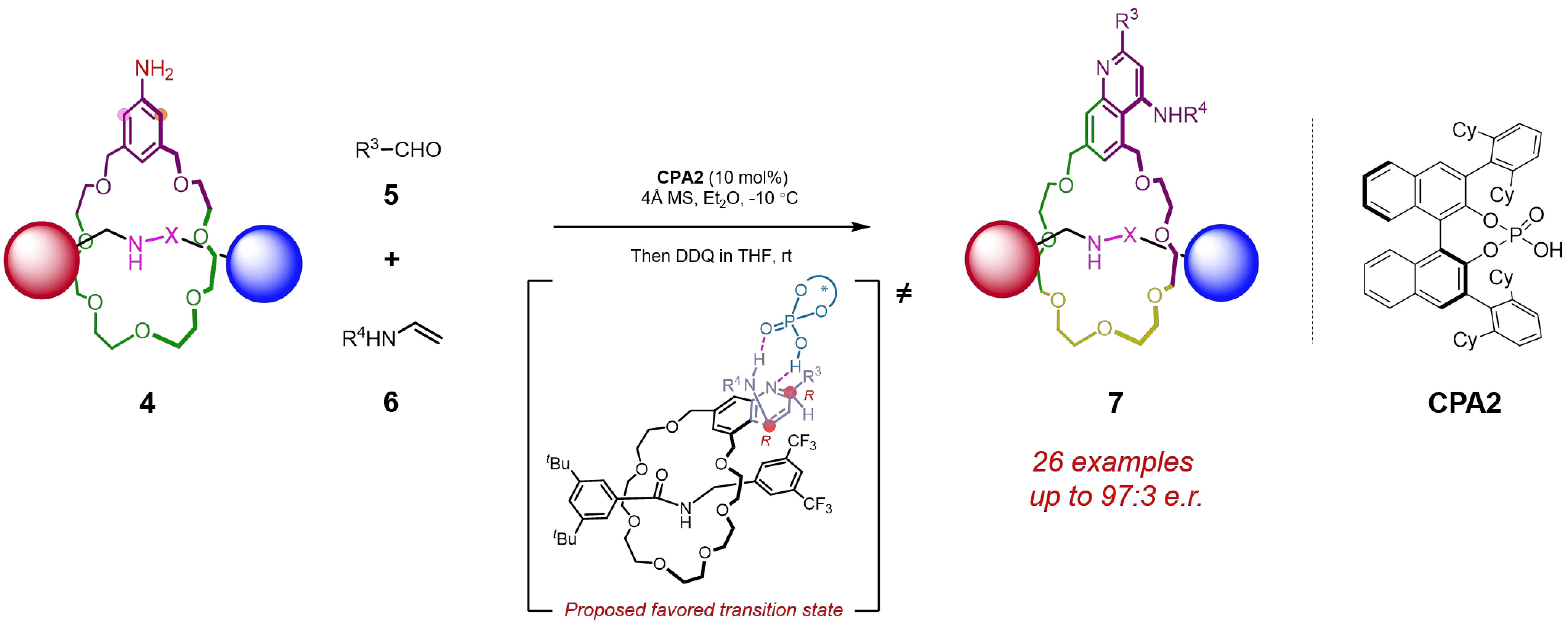
Figure 3. Enantioselective synthesis of MPC rotaxane via CPA-catalyzed three-component Povarov reaction. MPC: mechanically planar chirality; CPA: chiral phosphoric acid.
3. Conclusion
Although the concept of mechanically planar chirality was proposed as early as the 1970s, research on MPC rotaxanes remains relatively unexplored due to the limited methods available for accessing these molecules. Very recently, based on a desymmetrization strategy, the Yang group has developed elegant methodologies for the efficient asymmetric catalytic synthesis of MPC rotaxanes, demonstrating a broad substrate scope with high enantioselectivity. Nevertheless, as an emerging research area, further expansion of enantioselective synthesis strategies and functional applications for MPC rotaxanes is essential. Building on the current research landscape, several promising directions are proposed as follows. First, based on the desymmetrization strategy, additional reaction types that have proven effective for constructing chiral elements—such as asymmetric intramolecular/intermolecular C–H functionalization, aromatic sulfenylation, esterification, and related approaches—could be further explored for the enantioselective synthesis of MPC rotaxanes. Second, while current catalytic asymmetric methods predominantly employ crown ether-based macrocycles, the use of other types of macrocyclic components would significantly diversify the structures of accessible MPC rotaxanes. Third, the direct asymmetric catalytic conversion of prochiral or racemic rotaxanes represents an efficient route to access MPC rotaxanes, although prior synthesis of the rotaxane substrates is necessary. Therefore, methods that achieve stereocontrol simultaneously with mechanical bond formation from simple precursors are of considerable importance. Finally, the unique spatial structures and tunable chirality expression of MPC rotaxanes offer significant potential for applications in many areas. For instance, compared with conventional chiral elements, MPC rotaxanes possess extended and well-defined chiral cavities, offering a promising platform for the design of novel chiral ligands that could achieve enhanced stereoselectivity in asymmetric catalysis.[15] Furthermore, their interlocked topology and controllable molecular motion open up opportunities for unique applications in areas such as chiral luminescent materials, spintronics, and smart chiral materials. We believe that continued efforts in this field will contribute to its rapid advancement in the near future.
Authors contribution
Zhou XH: Investigation, visualization, writing-original draft.
Au-Yeung HY: Conceptualization, supervision, writing-review & editing.
Wang W: Conceptualization, project administration, funding acquisition, resources, supervision, writing-review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 22471076).
Copyright
© The Author(s) 2026.
References
-
1. Bruns CJ, Stoddart JF. The nature of the mechanical bond: From molecules to machines. Hoboken: John Wiley & Sons, Inc.; 2016.[DOI]
-
2. Jamieson EMG, Modicom F. Goldup SM. Chirality in rotaxanes and catenanes. Chem Soc Rev. 2018;47(14):5266-5311.[DOI]
-
3. Schill G. Catenanes, rotaxanes and knots. New York-London: Academic Press; 1971.[DOI]
-
4. Yamamoto C. Okamoto Y, Schmidt T, Jäger R, Vögtle F. Enantiomeric resolution of cycloenantiomeric rotaxane, topologically chiral catenane, and pretzel-shaped molecules: Observation of pronounced circular dichroism. J Am Chem Soc. 1997;119(43):10547-10548.[DOI]
-
5. Makita Y, Kihara Nobuhiro, Nakakoji N, Takata T, Inagaki S, Yamamoto C, et al. Catalytic asymmetric synthesis and optical resolution of planar chiral rotaxane. Chem Lett. 2007;36(1):162-163.[DOI]
-
6. Corra S, de Vet C, Groppi J, La Rosa M, Silvi S, Baroncini M, et al. Chemical on/off switching of mechanically planar chirality and chiral anion recognition in a [2]rotaxane molecular shuttle. J Am Chem Soc. 2019;141(23):9129-9133.[DOI]
-
7. Heard AW, Goldup SM. Synthesis of a mechanically planar chiral rotaxane ligand for enantioselective catalysis. Chem. 2020;6(4):994-1006.[DOI]
-
8. Gaedke M, Witte F, Anhäuser J, Hupatz H, Schröder HV, Valkonen A, et al. Chiroptical inversion of a planar chiral redox-switchable rotaxane. Chem Sci. 2019;10(43):10003-10009.[DOI]
-
9. Jinks MA, de Juan A, Denis M, Fletcher CJ, Galli M, Jamieson EMG, et al. Stereoselective synthesis of mechanically planar chiral rotaxanes. Angew Chem Int Ed. 2018;57(45):14806-14810.[DOI]
-
10. Tian C, Fielden SDP, Pérez-Saavedra B, Vitorica-Yrezabal IJ, Leigh DA. Single-step enantioselective synthesis of mechanically planar chiral [2]rotaxanes using a chiral leaving group strategy. J Am Chem Soc. 2020;142(21):9803-9808.[DOI]
-
11. Imayoshi A, Lakshmi BV, Ueda Y, Yoshimura T, Matayoshi A, Furuta T, et al. Enantioselective preparation of mechanically planar chiral rotaxanes by kinetic resolution strategy. Nat Commun. 2021;12:404.[DOI]
-
12. Li M, Chia XL, Tian C, Zhu Y. Mechanically planar chiral rotaxanes through catalytic desymmetrization. Chem. 2022;8(10):2843-2855.[DOI]
-
13. Tang M, Zhou J, Xie W, Ren J, Ye Z, Gu H, et al. Catalytic enantioselective synthesis of mechanically planar chiral rotaxanes by organocatalyzed desymmetrization. Chem Chem. 2026;12(1):102694.[DOI]
-
14. Ye Z, Xie W, Zhou J, Ma Y, Tang M, Ren J, et al. Enantioselective synthesis of mechanically planar chiral rotaxanes by an asymmetric Povarov reaction-enabled desymmetrization. Angew Chem Int Ed. 2025;64(51):e202515020.[DOI]
-
15. Martinez-Cuezva A, Saura-Sanmartin A, Alajarin M, Berna J. Mechanically interlocked catalysts for asymmetric synthesis. ACS Catal. 2020;10(14):7719-7733.[DOI]
Copyright
© The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite





