Abstract
Worldwide the cancer population is ageing-within a decade almost two-thirds of newly diagnosed patients will be aged 65 years and older. Despite this, the majority of oncology clinical trials continue to recruit patients who are younger and fitter than those typically encountered in clinical practice. As such, there is a lack of clinical data to guide management, particularly in those patients living with frailty and/or comorbidity. Importantly, the lack of older adults in trials also means that the subsequent translational work that underpins biomarker and therapeutic discovery may not be relevant to those we see in clinic. In this commentary, we discuss this challenge and the ways we as an Oncology community can look to address this pressing issue.
Keywords
1. Example
We are facing a pandemic of population ageing and growth, which is accompanied by a rising tide of cancer cases in older adults. By 2035, 60% of cancer diagnoses worldwide will be in patients aged older than 65[1]. Increasing age is associated with poorer cancer outcomes including survival and treatment tolerance[2]. Despite this, few oncology clinical trials specifically recruit older adults or have meaningful endpoints for an older population such as maintenance of independence or impacts on functional status and quality of life[3,4]. Those older adults recruited to clinical trials are often not representative and typically fitter compared with real-world populations[5].
Consequently, many treatment strategies have only been demonstrated to be beneficial in the younger, fitter populations traditionally recruited to clinical trials. Inappropriate use of or selection for treatments could cause greater harm than benefit, therefore decisions relating to systemic therapy, surgery or radiation must be made based on an assessment of both tumour biology and of the individual capacities of the older adult.
The lack of data relating to an older cancer population is a challenge to real-world clinical practice for several reasons. Firstly, it is difficult to accurately predict the impact of age on treatment tolerance for an individual patient, particularly in the context of the increasing use of complex multimodal and multiagent approaches. Age is associated with comorbidity and frailty[6], both of which are risk factors for poorer treatment tolerance[7].
To help clinicians, chemotherapy toxicity prediction tools are available and feasible for use in busy clinics[8,9], however they have limitations; they have only been validated for cytotoxics, they were developed in US-based populations only and individual patients’ toxicity risk may reflect multiple additional factors they do not capture. In addition, while these predictive tools used development populations which were balanced across older age groups, sex and comorbidity, they were primarily caucasian and of at least high school education. This may further limit their applicability to a wider population base.
Similar tools exist relating to complication risk from surgery in older adults (e.g. Vulnerable Elders Surgical Pathways and outcomes Assessment[10]) but no tool exists to predict either toxicity from radiotherapy alone or in combination with chemotherapy.
Secondly, there is an increasing appreciation that treatment efficacy may differ with age. For example, older adults appear to derive greater benefits from immune checkpoint inhibitors (ICI)[11]. These differences could be due to both age-related patient factors (e.g. drug pharmacokinetics and pharmacodynamics, reduced organ function and reserve, co-existing comorbidity or the impact of polypharmacy) or age-related differences in tumour biology and immunology. Recent studies have shown that older hosts (human and mice) have a more favourable tumour microenvironment for ICI response[12,13]. Benefits of treatment must always be balanced against the predicted life expectancy of an individual patient to help frame cancer treatment decisions.
While there have been several large studies in older adults investigating tools using patient factors to predict treatment toxicity and tolerance[9,14], as well as studies investigating the role of frailty screening and subsequent intervention on cancer outcomes[15-17], there has been little focus to date on the impact of age on tumour biology.
This is important as translational work derived from traditional clinical trials may not be representative of our real-world patients. As such, the current treatments we are using and identified potential biomarkers of outcome may not be personalised to our older
A growing body of evidence in the literature has demonstrated the differing biomarker profiles across a range of tumour groups[2]. These differences are not only observed using immunohistochemistry but also extend to differences at the genomic and transcriptomic level (Figure 1). However, data relating to these age-related differences is not available for all tumour sites, the number of patients included in studies is often small and response/survival data is often not available or powered to draw firm conclusions.
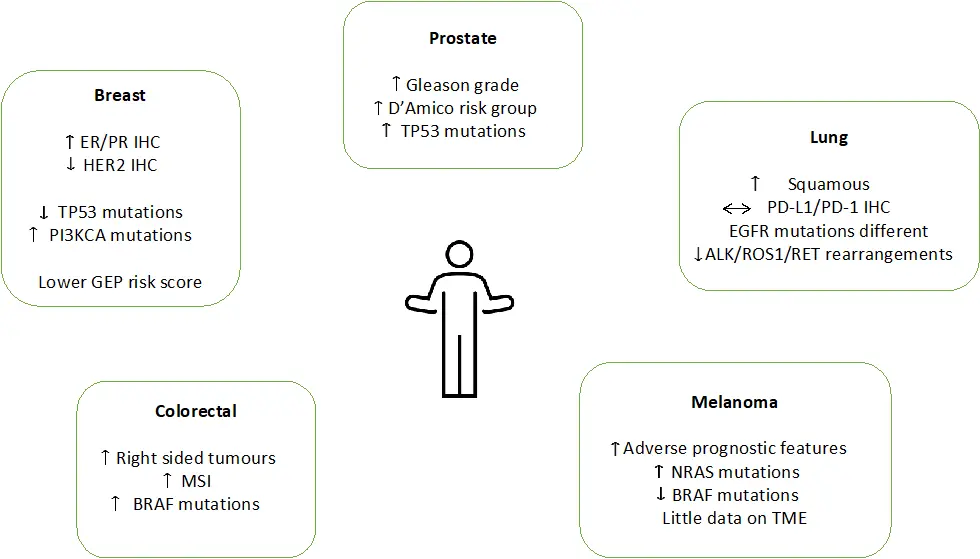
Figure 1. Differences in tumour biology and immunology with age in prostate, breast, colorectal, lung and melanoma. Adapted from Van Herck et al.[2]. EGFR: epidermal growth factor receptor; ER: oestrogen receptor; GEP: gene expression profile; MSI: microsatellite instability; PD-L1: programmed death ligand-1; PR: progesterone receptor; TME: tumour microenvironment.
The lack of data in older adults has been recognised by the American Society of Clinical Oncology and the International Society of Geriatric Oncology (SIOG) as a major challenge to cancer research and suggestions have been made regarding changes at every level of trial design[20,21].
To specifically address the gap in translational knowledge, we need to increase the number of prospective oncology trials designed for and collecting samples (both tissue and serum) in older adults. Exemplar’s of such an approach include the GO2 trial in advanced gastroesophageal cancer[22] and the currently recruiting FOXTROT2 study in early-stage colorectal cancer[23]. The translational work that emerges from these studies will provide vital insight into the potential differing tumour characteristics and biology that occurs in an older population. This will also provide an opportunity to identify novel biological targets as well as the ability to explore the overlap between tumour biology and patient biomarkers such as geriatric assessment and biomarkers of ageing.
While we wait, there is an opportunity for researchers to make use of stored tumour tissue from completed clinical trials, existing biorepositories of samples from real-world patients and registry databases to explore age-related differences both in tumours as well as treatment response and survival. Examples in breast cancer are the translational work by the Nottingham Breast Cancer
These steps are essential as we strive for a precision cancer medicine approach for our older adults—choosing the correct treatment for the correct patient at the correct time. Only by this approach, can we ensure our current and future treatments for our older adults with cancer are on target.
Authors contribution
Cheung KL, Battisti NML: Article conception, editing and approval of final manuscript.
Baxter MA: Article conception, drafting, editing and approval of final manuscript.
Conflicts of interest
All authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
None.
Copyright
© The Author(s) 2023.
References
-
1. Pilleron S, Sarfati D, Janssen‐Heijnen M, Vignat J, Ferlay J, Bray F, et al. Global cancer incidence in older adults, 2012 and 2035: a population‐based study. Rev Invest Clin. 2019;144(1):49-58.[DOI]
-
2. Van Herck Y, Feyaerts A, Alibhai S, Papamichael D, Decoster L, Lambrechts Y, et al. Is cancer biology different in older patients? Lancet Health Longev. 2021;2(10):e663-e677.[DOI]
-
3. Yang Y, Xie M, Zhang L, Yu K, Li H, Sun W, et al. Characteristics of older-patient-specif ic oncological trials: a cross-sectional analysis of ClinicalTrials. gov. Age Ageing. 2022;51(4):afac087.[DOI]
-
4. Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 2019;5(12):1769-1773.[DOI]
-
5. Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71(1):78-92.[DOI]
-
6. Hanlon P, Nicholl B I, Jani B D, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323-e332.[DOI]
-
7. Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091-1101.[DOI]
-
8. Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386.[DOI]
-
9. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.[DOI]
-
10. Pollock Y, Chan CL, Hall K, Englesbe M, Diehl KM, Min L. A novel geriatric assessment tool that predicts postoperative complications in older adults with cancer. J Geriatr Oncol. 2020;11(5):866-872.[DOI]
-
11. Wu Q, Wang Q, Tang X, Xu R, Zhang LZ, Chen X, et al. Correlation between patients’ age and cancer immunotherapy efficacy. Oncoimmunology. 2019;8(4):e1568810.[DOI]
-
12. Erbe R, Wang Z, Wu S, Xiu J, Zaidi N, La J, et al. Evaluating the impact of age on immune checkpoint therapy biomarkers. Cell Rep. 2021;36(8):109599.[DOI]
-
13. Garcia MG, Deng Y, Murray C, Reyes RM, Padron A, Bai H, et al. Immune checkpoint expression and relationships to anti‐PD‐L1 immune checkpoint blockade cancer immunotherapy efficacy in aged versus young mice. Aging Cancer. 2022;3(1):68-83.[DOI]
-
14. Extermann M, Boler I, Reich R, Lyman GH, Brown RH, DeFelice J, et al. The chemotherapy risk assessment scale for high-age patients (CRASH) score: design and validation. J Clin Oncol. 2010;28(15 suppl):9000.[DOI]
-
15. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904.[DOI]
-
16. Li D, Sun CL, Kim H, Soto-Perez-de-Celis E, Chung V, Koczywas M, et al. Geriatric Assessment–Driven Intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158.[DOI]
-
17. Lund CM, Vistisen KK, Olsen AP, Bardal P, Schultz M, Dolin TG, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Br J Cancer. 2021;124(12):1949-1958.[DOI]
-
18. Battisti NML, De Glas N, Soto-Perez-de-Celis E, Liposits G, Bringuier M, Walko C, et al. Chemotherapy and gene expression profiling in older early luminal breast cancer patients: an International Society of Geriatric Oncology systematic review. Eur J Cancer. 2022;172:158-170.[DOI]
-
19. Brain E, Viansone AA, Bourbouloux E, Rigal O, Ferrero JM, Kirscher S, et al. Final results from a phase III randomized clinical trial of adjuvant endocrine therapy ± chemotherapy in women ≥ 70 years old with ER+ HER2- breast cancer and a high genomic grade index: the Unicancer ASTER 70s trial. J Clin Oncol. 2022;40(16 suppl):500.[DOI]
-
20. Soto-Perez-De-Celis E, Lichtman SM. Considerations for clinical trial design in older adults with cancer. Expert Opin Inv Drug. 2017;26(10):1099-1102.[DOI]
-
21. Hurria A, Levit LA, Dale W, Mohile SG, Muss HB, Fehrenbacher L, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33(32):3826-3833.[DOI]
-
22. Hall PS, Swinson D, Cairns DA, Waters JS, Petty R, Allmark C, et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869-877.[DOI]
-
23. Platt JR, Williams CJM, Craig Z, Cairns DA, Glasbey JC, Morton D, et al. Personalizing neoadjuvant chemotherapy for locally advanced colon cancer: protocols for the international phase III FOxTROT2 and FOxTROT3 randomized controlled trials. Colorectal Dis. 2023;25(3):357-366.[DOI]
-
24. Syed BM, Green AR, Paish EC, Soria D, Garibaldi J, Morgan J, et al. Biology of primary breast cancer in older women treated by surgery: with correlation with long-term clinical outcome and comparison with their younger counterparts. Br J Cancer. 2013;108(5):1042-1051.[DOI]
-
25. Syed BM, Green AR, Rakha EA, Morgan DAL, Ellis IO, Cheung KL, et al. Age-related biology of early-stage operable breast cancer and its impact on clinical outcome. Cancers. 2021;13(6):1417.[DOI]
-
26. Parks RM, Albanghali MA, Syed BM, Green AR, Ellis IO, Cheung KL. Patterns of biomarker expression in patients treated with primary endocrine therapy–a unique insight using core needle biopsy tissue microarray. Breast Cancer Res Treat. 2021;185(3):647-655.[DOI]
-
27. Depoorter V, Vanschoenbeek K, Decoster L, De Schutter H, Debruyne PR, De Groof I, et al. Linking clinical and population-based data in older patients with cancer in Belgium: feasibility and clinical outcomes. J Geriatr Oncol. 2023;14(2):101428.[DOI]
Copyright
© The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




