Abstract
Immune cell aging is associated with compromised cancer immunosurveillance and reduced efficacy of some cancer immunotherapies. The ability to reverse immune cell aging to obtain more efficient anti-tumour reactive T cells would provide obvious benefits in clinical treatment. This could be achieved by acting on key fundamental cellular processes including metabolism and autophagy, which may subsequently influence T cell differentiation and effector functions. Polyamines, which can induce autophagy, have been shown both to enhance mitochondrial activity and have a direct effect on T cells. First by improving effector functions in CD8 T cells and, second, by regulating CD4 T cell differentiation. However, the exact interconnections between autophagy, mitochondrial activity and T-cell function remain to be elucidated. Most of the data on these fundamental processes have been collected from non-human systems, but fewer clinical data are available. We herein discuss the main evidence and speculate on the eventual benefit of affecting metabolism in an aged immune system to improve immunotherapy outcome.
Keywords
1. Introduction
The role of the immune system in preventing cancer is well recognized, however, as any organ, this system is compromised by aging which can reduce its capacity to control cancer development and affect treatments in general[1].
An aging adaptive immune system is characterized by increasing malfunction and autoimmunity, probably resulting from a change in immune cell composition[2] and a loss in quality of immune response. Several hallmarks of T cell aging have been described, including thymic involution, loss of proteostatis, genetic alterations, inflammaging, T cell receptor (TCR) repertoire reduction, lack of plasticity and naïve- memory imbalance, T cell senescence, and mitochondrial dysfunction[2,3]. The aging immune system generally responds more poorly to vaccination (flu, Covid), however, in some cases age seems to have no effect on treatment outcome as recently reported for CD19 chimeric antigen receptor (CAR) T cell treatment of B-cell lymphoma[4]. Surprisingly, certain types of immunotherapies like immune checkpoint inhibitors seem to be as, or even more, efficient, in older patients[5,6], but this represents an exception. The reasons for these discrepancies in treatment response are not fully understood. Preclinical data suggest that the composition of T cell populations might be the cause: a higher Treg to CD8 T cell ratio in younger patients, or an enhanced memory to naïve T cell ratio in elderly patients, which remains plausible. On the other hand, certain age-associated traits of the immune system have also been associated with increased risk of adverse events[7]. In murine cancer models, CD4 T cells in aged mice treated with anti-PD-1 causing immune-related adverse events, were found to secrete more IL-21 which upregulated the B-cell homing chemokine CXCL13 and lead to autoimmune pathogenicity[7]. Systemic increase of CXCL13 and CD4 T cell-associated IL-21 expression also correlated with immune-related adverse events in patients.
The tumour microenvironment (TME) plays an essential role in tumour growth and therapy resistance. Numerous non-malignant cells in the tumour stroma, e.g. immune cells, fibroblasts, endothelial cells, contribute to tumour progression. Aging also clearly influences the behaviour of the TME[8].
The effects of aging are very rarely considered when performing preclinical experiments and novel immunotherapies may therefore not be designed in an optimal manner for older patients who represent the majority of cancer patients treated.
2. Can We Simply Reverse the Aging of the Immune System to Improve the Effect of Cancer Treatment?
Autophagy has received considerable attention in recent years in connection with aging and disease. It is a cellular process through which damaged or dysfunctional cellular components are broken down and recycled. These resulting subproducts can then be used as fuel to produce catabolic energy. Thus, autophagy is mainly a protective activity to cells and compromised autophagy is a hallmark of aging. Autophagy has tissue-specific roles in regulating aging and tissues degenerate at different pace[9].
Autophagy, and in particular the balance between mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) activation, is essential in regulating the differentiation and metabolism of immune cells. When T cells are activated and receive co-stimulation through CD28, the PI3K-AKT-mTOR signalling, the autophagic flux is reduced and accompanied by increased cellular glycolytic activity, inducing T cell proliferation and a pro-inflammatory phenotype[10]. Memory T cells continue to depend on this CD28-mediated signalling pathway, but also use additional mechanisms to increase glycolysis. Moreover, memory cells take up glucose faster than naïve cells and more efficiently use this glucose for fatty acid synthesis when rechallenged with antigen[11].
Highly glycolytic activated T cells cannot maintain cellular energy levels in environments with low glucose availability such as the TME. Consequently, in order to proceed to efficient catabolic metabolism, T cells need to dip into their reserves. This will be executed by activating the key metabolic regulator AMPK which will stimulate autophagy through inhibition of mTOR, and promote the oxidative phosphorylation (OXPHOS)-dependent function of non- or anti-inflammatory T cells. Since autophagy is affected in aged T cells, their responsiveness upon activation might be more affected by a hostile TME.
Thus, it is legitimate to ask whether autophagy could be used to metabolically regulate and protect anti-tumour immune cells in cancer treatment in vivo or for ex vivo production of more efficient, and also more persistent therapeutic cells.
Modulating the T cell metabolism is a clear aim to improve anti-tumour T cell function; however, whether this can be done through simply increasing autophagy remains an open question. Drugs modulating autophagy are likely to also exert other effects on immune cells.
There are several ongoing therapeutic efforts to modulate autophagy and delay aging. Different types of interventions including dietary restriction, exercise and supplementation with small chemical compounds, such as spermidine, have been shown to stimulate autophagy[9]. The majority of this evidence, however, comes from experiments in yeast, flies, worms and rodents. Although autophagy is a fundamental cellular process that is highly conserved between species, this still requires further testing in humans for successful clinical interventions.
3. Enhancing the Anti-tumour Effect of T Cells
A recent article showed that a naturally occurring polyamine, spermidine, which is also known to be an activator of autophagy, improved CD8 T cell anti-tumour function in aging mice[12]. Spermidine levels decrease with age, and spermidine supplementation in animals has been shown to improve anti-tumour immunity, but here Al-Habsi and colleagues showed that spermidine addition synergized with anti-PD-L1 treatment and enhanced CD8 T cell tumour infiltration, proliferation, and IFN-γ secretion in vivo in tumour-bearing mice. They isolated naïve and memory CD8 T cells from tumour draining lymph nodes and showed that the spermidine addition enhanced mitochondrial activity. Using a Seahorse XF instrument (Agilent Technologies), the authors measured the oxygen consumption rate and extracellular acidification rate reflecting OXPHOS and glycolysis, respectively. CD8 T cells from the TDLN of aged mice treated with spermidine and anti-PD-L1 demonstrated increased OXPHOS and mitochondrial ATP production.
Despite the observed changes in mitochondrial activity and that spermidine is known to increase autophagy, the authors did not detect an increase in autophagy-related proteins or changes in mitochondrial morphology one hour after addition, however, this may have been too early. Using mice with T-cell specific deletion of a subunit of the mitochondrial trifunctional protein, the enhancing effects of spermidine on fatty acid oxidation and anti-tumour immunity were prevented. This study provided additional mechanistic insight into the effect of spermidine on CD8 T cells. However, whether this is ultimately due to autophagy and its anti-aging properties or also dependent on other mechanisms is not yet clear. The anti-tumour effects of spermidine supplement in vivo are likely to depend on a range of additional mechanisms, including direct effects on cancer cells, reduced inflammaging, and increased T cell stemness.
SmartAge, a randomized, placebo-controlled trial in Germany, tested the effect of long-term spermidine supplement in 100 individuals of 60-90 years old with cognitive decline[13]. Possible beneficial effects on verbal memory and inflammation were observed in exploratory analyses, but spermidine supplementation did not otherwise induce any significant changes in memory performance or any other neuropsychological, behavioral, or physiological parameters measured. These individuals did not have cancer, and any positive effects on memory and inflammation will have to be validated in future studies at higher dosages.
A recent pre-clinical study, however, tested how in vitro pretreatment of CART cells with spermidine affected their phenotype and anti-lymphoma efficacy in vitro and in vivo[14]. Nevertheless, as mentioned before, aging was not correlated to reduced response in lymphoma CAR therapy[4], and these experiments should be repeated with CARs targeting solid tumours, preferentially in the presence of a strongly immunosuppressive TME. Although preliminary, the results of this study indicated that spermidine pre-treatment could enhance the CART cell cytotoxicity and provide a higher proportion of central memory T cells, in line with previous reports[15].
In contrast to these above results, a report published during the preparation of this manuscript showed that tumour cell-derived spermidine inhibited CD8 T cell anti-tumour responses through downregulation of cholesterol in the cell membrane, thereby reducing TCR clustering[16]. The contradictory results may be related to the doses of spermidine used in the different studies and warrant further investigation.
Another downside of applying drugs that increase autophagy in cancer patients is that the effects are not necessarily specific. These drugs can also act on cancer cells to augment autophagy, leading to increased tumour growth and therapy resistance [17]. It may therefore be a preferable option to treat T cells ex vivo with autophagy increasing drugs prior to administering T cell based therapies.
4. What about CD4 T Cells?
As an additional layer to this complexity, the cancer immunotherapy field has traditionally mostly focused on CD8 T cells. The role of CD4 T cells has primarily been thought to mediate anti-tumour immunity by providing help for CD8 CTL and antibody responses. Nevertheless, CD4 T cells are the main organizers of adaptive immune responses and it has become increasingly clear that CD4 T cells play a critical role in not only developing and sustaining effective anti-tumour immunity, but also as anti-tumor effector cells in their own right[18,19]. CD4 T helper (Th) cells are plastic and can differentiate into multiple subsets (e.g. Th1, Th2, Th17, Treg) with various functions to regulate immunity against various types of infections, or cancer. With age, there is a change in plasticity and CD4 T cells seem to be pushed towards extreme regulatory and effector phenotypes[20]. Age-associated clonal expansion of cytotoxic CD4 T cells has been identified in human supercentenarians and could thus be an adaptation of the immune system to achieve exceptional longevity[21]. As CD4 T cells regulate immunity, they also promote aging in other immune cell compartments and regulating their phenotype and function could broadly affect immunity.
Spermidine was recently shown to regulate the metabolism and anti-tumour functions of CD8 T cells[12], but polyamine metabolism has also been shown to control the ability of CD4 T cells to polarize into different functional subsets[22]. During polyamine synthesis, the rate limiting enzyme ornithine decarboxylase convert the amino acid ornithine into putrescine which can then be metabolized to the polyamines spermidine and eventually spermine. Puleston and colleagues showed that loss of polyamine synthesis profoundly affected the CD4 Th lineage commitment[22]. They found that polyamine influx from the microenvironment and the levels of various cytokines present during activation may contribute significantly to intracellular polyamine levels. They also found that histone acetylation played a key role in how polyamine metabolism regulated the T cell epigenome which they suggested to be driven through the tricarboxylic acid cycle. Also acting through inhibition of histone deacetylases, lactate was recently found to increase stemness in CD8 T cells with an increase in the transcription factor TCF-1[23]. Puleston and colleagues further found that polyamine metabolism limited ectopic expression of Th cell lineage transcription factors and cytokines, and therefore reduced polyamine synthesis or bioavailability in disease settings or in aging, could promote lack of CD4 Th cell plasticity, pushing the cells into more extreme regulatory and effector phenotypes[20]. The effect of polyamine supplementation on CD4 T cells subsets in vivo is still unclear, however[24].
5. Conclusions
The induction of autophagy or a metabolic switch to increased fatty acid oxidation by supplementation of polyamines like spermidine could be an important way to achieve longevity and to enhance anti-tumour immunity.
Exactly how the processes of autophagy, polyamine metabolism and T cell function are linked still needs to be further studied. Most of these fundamental processes have been evaluated in non-human models.
Even in the same cell lineage, we know that essential metabolic processes can play different roles depending on the functional program they initiate and their microenvironment.
There are few controlled studies on polyamine supplementation in humans and in cancer patients. It is so far unknown if exogenous addition of polyamines or other metabolic modulators (Figure 1) can just temporarily modulate T cell metabolism and induce autophagy to improve immune cell effector functions, or if this could actually rejuvenate T cells more permanently. Other signs of reduced aging in these T cells should be investigated, including phenotype with differentiation markers, poor response to stress, epigenetic alterations, and telomere attrition.
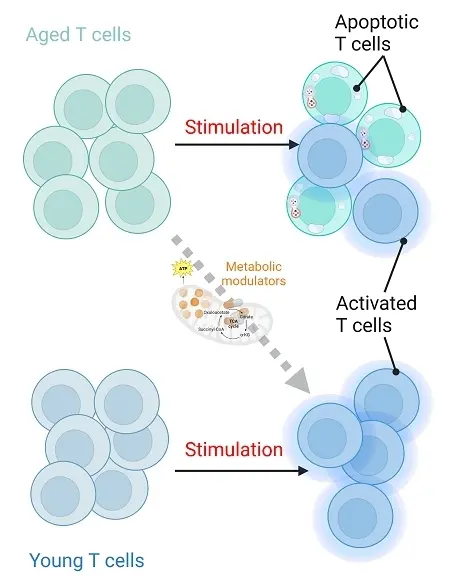
Figure 1. Rejuvenation and enhanced activation of immune cells by metabolic modulators. Created in Biorender.com.
Treating CAR- or TCR-modified T cells with metabolic modulators prior to injection into patients is an appealing idea, but this may not sufficiently boost the cells with improved effector functions or resistance to a solid TME to see clinical differences. However, if this could lead to more permanent changes in T cell stemness, it could have great potential to improve adoptive T cell therapy.
Authors contribution
All authors contributed equally to this work..
Conflicts of interest
Else Marit Inderberg is an Editorial Board member of Ageing and Cancer Research & Treatment. Sébastien Wälchli declares no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was partially supported by the National Centre for Research and Development within POLNOR program ALTERCAR (#NOR/POLNOR/ALTERCAR/0056/2019), EURONANOMED-3 NAN-4-TUM (NFR#310531) and the KSP-2021 funding from the Research Council of Norway for the CellFit project (#326811).
Copyright
© The Author(s) 2023.
References
-
1. Foster AD, Sivarapatna A, Gress RE. The aging immune system and its relationship with cancer. Aging Health. 2011;7(5):707-718.[DOI]
-
2. Han S, Georgiev P, Ringel AE, Sharpe AH, Haigis MC. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023;35(1):36-55.[DOI]
-
3. Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687-698.[DOI]
-
4. Dreger P, Holtick U, Subklewe M, von Tresckow B, Ayuk F, Wagner E, et al. Impact of age on outcome of CAR-T cell therapies for large B-cell lymphoma: the GLA/DRST experience. Bone Marrow Transplant. 2023;58(2):229-232.[DOI]
-
5. McElhaney JE, Verschoor CP, Andrew MK, Haynes L, Kuchel GA, Pawelec G. The immune response to influenza in older humans: beyond immune senescence. Immun Ageing. 2020;17:10.[DOI]
-
6. Newman J, Thakur N, Peacock TP, Bialy D, Elrefaey AME, Bogaardt C, et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat Microbiol. 2022;7(8):1180-1188.[DOI]
-
7. Tsukamoto H, Komohara Y, Tomita Y, Miura Y, Motoshima T, Imamura K, et al. Aging-associated and CD4 T-cell–dependent ectopic CXCL13 activation predisposes to anti–PD-1 therapy-induced adverse events. Proc Natl Acad Sci USA. 2022;119(29):e2205378119.[DOI]
-
8. Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89-106.[DOI]
-
9. Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1(8):634-650.[DOI]
-
10. Shyer JA, Flavell RA, Bailis W. Metabolic signaling in T cells. Cell Res. 2020;30(8):649-659.[DOI]
-
11. Puleston DJ, Simon AK. New roles for autophagy and spermidine in T cells. Microb Cell. 2015;2(3):91-93.[DOI]
-
12. Al-Habsi M, Chamoto K, Matsumoto K, Nomura N, Zhang B, Sugiura Y, et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science. 2022;378(6618):eabj3510.[DOI]
-
13. Schwarz C, Benson GS, Horn N, Wurdack K, Grittner U, Schilling R, et al. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline: a randomized clinical trial. JAMA Netw Open. 2022;5(5):e2213875.[DOI]
-
14. Wang H, Jiang D, Liu L, Zhang Y, Qin M, Qu Y, et al. Spermidine promotes Nb CAR-T mediated cytotoxicity to lymphoma cells through elevating proliferation and memory. OncoTargets Ther. 2022;15:1229-1243.[DOI]
-
15. Puleston DJ, Zhang H, Powell TJ, Lipina E, Sims S, Panse I, et al. Autophagy is a critical regulator of memory CD8(+) T cell formation. Elife. 2014;3:e03706.[DOI]
-
16. Hibino S, Eto S, Hangai S, Endo K, Ashitani S, Sugaya M, et al. Tumor cell-derived spermidine is an oncometabolite that suppresses TCR clustering for intratumoral CD8+ T cell activation. Proc Natl Acad Sci USA. 2023;120(24):e2305245120.[DOI]
-
17. Lim J, Murthy A. Targeting autophagy to treat cancer: challenges and opportunities. Front Pharmacol. 2020;11:590344.[DOI]
-
18. Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell. 2020;181(7):1612-1625.[DOI]
-
19. Cachot A, Bilous M, Liu YC, Li X, Saillard M, Cenerenti M, et al. Tumor-specific cytolytic CD4 T cells mediate immunity against human cancer. Sci Adv. 2021;7(9):eabe3348.[DOI]
-
20. Elyahu Y, Hekselman I, Eizenberg-Magar I, Berner O, Strominger I, Schiller M, et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci Adv. 2019;5(8):eaaw8330.[DOI]
-
21. Hashimoto K, Kouno T, Ikawa T, Hayatsu N, Miyajima Y, Yabukami H, et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc Natl Acad Sci USA. 2019;116(48):24242-24251.[DOI]
-
22. Puleston DJ, Baixauli F, Sanin DE, Edwards-Hicks J, Villa M, Kabat AM, et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell. 2021;184(16):4186-4202.[DOI]
-
23. Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, et al. Lactate increases stemness of CD8+ T cells to augment anti-tumor immunity. Nat Commun. 2022;13(1):4981.[DOI]
-
24. Fischer M, Ruhnau J, Schulze J, Obst D, Flöel A, Vogelgesang A, et al. Spermine and spermidine modulate T-cell function in older adults with and without cognitive decline ex vivo. Aging. 2020;12(13):13716-13739.[DOI]
Copyright
© The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




