Abstract
Over the past 50 years, the world has experienced a progressive demographic shift resulting in a higher proportion of older adults in the general population. Aging itself is a complex biological phenomenon, characterized in part by changes in the immune system, known as “immunosenescence”, that make older adults more susceptible to infections, cardiovascular and autoimmune diseases, and cancer. Several strategies have been proposed to reverse immunosenescence. These include the use of hormones, cytokines, and thymic factors. Biomodulina T (BT) is a polypeptide fraction derived from bovine thymus. Intervention with BT expands naïve CD4+ T cells while decreasing the frequency of CD4+ and CD8+ T cells expressing PD1 in older adults and patients diagnosed with advanced lung cancer. This brief review aims to present the most recent evidence on the effect of BT on the immune system in older adults and patients diagnosed with advanced lung cancer.
Keywords
1. Introduction
In the coming years, the number of older adults is projected to exceed the number of children for the first time in recorded history. Latin America, and Cuba in particular, are also expected to see a large increase. Cuba has one of the oldest populations in the Americas[1]. The aging of an individual is known to be associated with functional changes in immunity resulting from age-related alterations in both the innate and adaptive branches of the immune system[2]. Dysregulation of the immune system with age is commonly referred to as “immunosenescence”. Cellular senescence, an irreversible exit from the cell cycle due to aberrant or extensive replication or different types of damage and stress, including genotoxic and oxidative damage and inflammatory stress, may be associated with some manifestations of immunosenescence. Of note, senescent cells acquire an inflammatory senescence-associated secretory phenotype which comprises heterogeneous constellations of several families of soluble factors such as interleukins, chemokines, growth factors and proteases, as well as extracellular matrix components. These may contribute to the commonly observed low level chronic inflammatory state referred to as “inflammaging”[2,3], thought to mediate some aspects of organismal aging[2,4-6]. Positive feedback between immunosenescence and inflammaging may exacerbate the effects of both, and help to explain older adults’ particular susceptibility to new infections and chronic disease, including cardiovascular, metabolic, neurodegenerative and cancer[1,4].
The growing prevalence of cancer is a major health problem around the world. The global burden of cancer is projected to be on the rise for at least the next 2 decades[7]. Recently it has been proposed that both aging and cancer are characterized by partially overlapping “hallmarks”; these shared characteristics are referred to as “meta-hallmarks”. Indeed, more than 60% of newly diagnosed cancer patients and more than 70% of cancer-related deaths occur in older adults[8]. Due to their comorbidities and different functional status, older patients present a heterogeneous population. This makes them more challenging for oncologists and scientists seeking personalized cancer therapies[9].
Several strategies have been proposed to reverse the changes that occur in the immune system with age and thus potentially contribute to improving quality of life in older adults. The critical question is if the process of immunosenescence can be therapeutically modified. In such a case, immunosenescence could become a target for therapeutic intervention, beyond the specific molecular targets related with oncogenic transformation. One of the proposed strategies is devoted to restoring and maintaining a normal thymic microenvironment and is defined as “restoration”, as part as the so called “3Rs of immune rejuvenation”[10]. It is postulated that thymic rejuvenation would increase naïve T cell generation, hence increasing the T cell antigen receptor repertoire and facilitating the recognition of cancer neoantigens[11,12].
In the present paper we review the current and most recent knowledge regarding the effect of Biomodulina T on the immune system in older adults[13] and in patients diagnosed with lung cancer treated with chemotherapy[14].
2. Aging, Cancer and the Immune System
Aging is the single most important risk factor for several non-communicable disease of the adulthood and, by definition, of the geriatric syndromes. However, the aging process varies widely among individuals, which means that aging is not uniform from person to person[2]. The most studied changes in the immune system with age consist of those that occur in the adaptive branch. Although the use of specific biomarkers to characterize immunosenescence is intensely debated, there is consensus on the decrease of naïve cells and the increase of memory (senescent/exhausted) cells, principally in the so-called terminally-differentiated T cells[15] which downregulate membrane expression of the CD28 coreceptor[16], and re-express the CD45RA marker[17].
As a consequence of thymic involution, beginning at puberty, reserves of naïve T cells are depleted throughout life, and additionally due to the conversion of naïve cells to memory cells over a lifetime of exposure to immune challenges (pathogens, but potentially including autoantigens and cancer antigens)[18]. There is considerable evidence to suggest that chronic antigenic stimulation, induced by the presence of persistent infections or by altered tissues and molecules, plays an important role in driving the peripheral T cell compartment into a state that is different in older individuals. The accumulation of late-stage differentiated CD8+ T cells specific for cytomegalovirus (CMV) is a classic example of this process, which is caused by this latent herpesvirus infection[19]. As age increases, the proportion of the population infected with CMV increases in most countries, which eventually includes the majority of older people[20]. Although most of the literature on immunosenescence has focused on T cell changes with age, other immune compartments such as B cells and innate populations such as NK cells and dendritic cells also differ between older and younger people[15,21].
Most types of cancer can be considered age-related diseases, suggesting that the mechanisms of control of aging and cancer are intimately related[8]. In a previous study, our group has reported a cancer-associated decrease in the CD4/CD8 ratio and higher percentage of patients with an inverted CD4/CD8 ratio in patients diagnosed with advanced lung cancer, but not in age and sex-matched healthy volunteers. In addition, we described a greater proportion of terminally differentiated (CD280-) CD4+ and CD8+ T cell subpopulations in patients treated with first-line platinum-based chemotherapy[9]. Collectively, this evidence suggested that advanced cancer and chemotherapy adversely affect the immune status of these patients. In addition, many other factors that play a role in the pathogenesis of cancer, such as low-grade inflammation, decreased immunosurveillance, apoptosis resistance, and others, also become more prevalent as part of the aging process[8].
Cancer immunotherapy has continued to evolve, with several forms of treatment advancing. However, the most convincing evidence that the immune system can control cancer has come from the outstanding clinical success of immune checkpoint inhibitors (ICI). Nevertheless, a proportion of patients treated with ICI fails to respond to this promising therapy, demonstrating that tumors escape immunity. A lack of tumor-specific T cells, which may be a consequence of immunosenescence, may be one possible explanation[22].
In addition to checkpoint blockade immunotherapies to boost anti-cancer T cell responses, several avenues have been explored to improve T cell function and restore a normal thymic environment in the elderly population[13]. These strategies include non-pharmacological approaches such as physical activity and healthy nutrition, but also the use of hormonal products, growth factors, cytokines and thymic peptides[15].
Lang and colleagues proposed the 3Rs of rejuvenation: restoration, replacement and reprograming strategies. The restoration strategy is defined as the preservation of a normal thymic environment by the administration of growth hormone, sex steroids, growth factors, nutrients, and cytokines[10]. Although there may be potential negative consequences of rejuvenating an organ selected by evolution to involute with age, thymic regeneration and also age-specific immune interventions, certainly could contribute to preserve/restore immune protection, and to potentiate the immune system to better resist infections and cancer, likely relevant for the aging population.
3. Biomodulina T
Since the second half of the last century, several thymus-derived pharmaceuticals have been studied for the treatment of primary immunodeficiencies and other diseases and conditions such as cancer[23,24]. Thymus-derived products can be divided into two groups: (i) purified extracts from animal thymus glands containing peptide mixtures, and (ii) synthetically produced thymic peptides[23]. Extracts from calf thymus are further processed through various steps of purification, fractionation, and filtration to produce mixtures of peptides. Of particular note is thymosin fraction 5, which was first described in 1966 and has been extensively studied in clinical trials[25].
Biomodulina T is another example of natural immunomodulator composed of polypeptide fractions obtained from the bovine thymus (Centro Nacional de Biopreparados (BIOCEN), Havana, Cuba). Development of this product began in 1984, followed by a series of experiments confirming its biological activity. An extensive series of toxicological studies were also performed, confirming that it is a safe thymic factor. Several clinical trials have been conducted in patients with immunodeficiency disorders, recurrent infections, autoimmune diseases, cancer and other diseases[26]. In 1994, the Cuban Regulatory Agency granted a license for the treatment of recurrent infections in elderly patients[27].
When BT was used in elderly patients with Chronic Obstructive Pulmonary Disease, it was reported a decrease in bronchopneumonia recurrences in these patients[13,28]. After treatment with BT in children with thymic atrophy or hypoplasia, the recovery of thymic mass evaluated by thymic echography, as well as the reduction of recurrent infectious processes have been reported[29]. In a clinical trial in patients with relapsing-remitting multiple sclerosis, clinical parameters improved and immunological parameters normalized and remained normal after BT administration[30]. More recently, Biomodulina T has also been used during the covid outbreak and has been shown to contribute to reduction of mortality in aged patients[31]. In addition, BT induced immune restoration of T and B lymphocyte populations in institutionalized older adults[32], as well as expansion of B and NKT subsets when administered at a lower dose of 3 mg three times a week for one week to older adults[33].
BT has very low toxicity, which is supported by the preclinical and clinical studies presented in the registration dossier[27]. All of the clinical studies mentioned above confirm the safety profile of this drug[1,13], with pain and burning at the injection site, fever, headache and asthenia being the most common side effects reported[27].
4. Biomodulina T Partially Restores CD4+ and CD8+ T Cell Compartments in Older Adults
Several strategies have been investigated to improve thymopoietic potential and reduce the effects of thymic involution. These treatments enhance thymic output and induced expansion of naïve T cells[34]. Therapies such as zinc supplementation, leptin treatment, keratinocyte growth factor administration, sex steroid ablation, and citokine administration have been studied in animals and in various clinical scenarios[15,34,35]. Human clinical trials for IL7 demonstrated the expansion of peripheral T cells including the naïve T cell compartment[36] as well as higher TCR diversity and augmentation of effector memory cells, with no effect on T regulatory cells[37].
A study conducted in older adults with a history of recurrent respiratory infections (and no other previously diagnosed chronic disease), revealed that BT transiently expanded naïve CD4+ T cells, recent thymic emigrants (RTE), and stem cell-like memory CD8+ T cell production, and also decreased the proportions of CD4+PD1+ and CD8+PD1+ T cells, suggesting a potential “anti-exhaustion” effect of BT for the immune response of older people[13]. Additionally, BT treatment increased the expression of the Ki67 nuclear marker in CD4+ T cells, which was interpreted as an enhancement of the proliferative capacity of helper T cells in older adults induced by this thymic factor. There was also an increase in the intracellular expression of IFN-γ, indicating that BT may have a role in the restoration of the Th1 response in older adults[13].
All of the immune benefits of BT described above occur in the absence of regulatory T cell expansion. This is noteworthy because BT would be expected to stimulate thymic production of several different cellular subpopulations, including natural regulatory T cells[13].
Taken together, these results pointed to the impact of BT as a promising strategy for immune restoration in older adults and for enhancing the immunotherapeutic potential in cancer patients.
5. Biomodulina T Decreases Exhausted and Terminally-differentiated EMRA T Cells in Advanced Lung Cancer Patients Treated with Platinum-based Chemotherapy
Impairment of the immune system after chemotherapy has been well studied[9,38,39]. In previous work, our group has shown that chemotherapy significantly reduces the frequency of naïve and early-differentiated T cells and increases late-stage T cell populations[40]. Recovery of immune populations after chemotherapy has been reported to be variable, usually slow and progressive[39]. Naïve CD8+ T cells increased and terminally differentiated effector T cells normalized after 12 months in patients treated with docetaxel and cyclophosphamide[41]. Similarly, thymic recovery, as assessed by CD31+RTE T cells, was observed 1 year after the end of treatment in patients diagnosed with Hodgkin lymphoma and B-cell lymphoma[42]. Another study, conducted in patients with multiple cancer sites and treatment types, found that B and NK cells have recovered to pretreatment levels in as little as 3 months, while overall T cell compartment recovery occurred 6 months after treatment, with the exception of helper and naïve subpopulations, which recovered on average by 12 months after chemotherapy[43].
In a recent work, BT was administered (intramuscularly three times a week for four weeks) to patients diagnosed with advanced lung cancer, five to seven days after completing first-line platinum-based chemotherapy. Terminally differentiated T cells (CD4+EMRA and CD8+EMRA) decreased after BT treatment while CD4+ naïve T cells increased, confirming the ability of BT to expand naïve or early differentiated T cells in the periphery. In addition, CD4+ and CD8+ T cells expressing PD1 were reduced after BT administration, highlighting its likely effects countering “exhaustion” not only in healthy older adults[13] but also in cancer patients[14].
Five to seven days after finishing BT administration, patients started treatment with CIMAvax-EGF[14]. This is an EGF-depleting immunotherapy that induces neutralizing anti-EGF antibodies that recognize circulating EGF, preventing it from binding to EGFR, and thereby disrupting the signal transduction cascade associated with proliferation and survival signals in tumor cells[44]. The sequential combination treatment of BT and CIMAvax-EGF was found to be safe; as previously reported, neither BT nor CIMAvax-EGF were related to grade 3 or 4 adverse events (AE) and no serious AE were observed[14]. The proposal of sequential combination of BT and CIMAvax-EGF, both after completion of first-line chemotherapy, administered as switch maintenance, benefited a larger percentage of patients than previously described for CIMAvax-EGF alone, met the good antibody response criteria (i.e. developed anti-EGF antibody titers equal or higher than 1:4,000[45]). Therefore, the combination was successful and enhanced the specific anti-EGF antibody response[14].
6. Conclusions
Approaches to immune “rejuvenation” in older adults may be necessary, despite the likelihood that many of the hallmarks of “immunosenescence” reflect the adaptive nature of immunity. Given that natural evolution is unlikely to promote substantial survival beyond reproductive age, and that solid cancers expressing neoantigens mostly occur at older age, one mechanism contributing to failed immunosurveillance may be immunosenescence. In such a case, further strategies to improve the immune response in older adults may be desirable. This is of particular interest to enhance the immunotherapeutic potential in patients diagnosed with cancer, where the immune system is further compromised by chronic disease. Administration of BT helps to improve the immune response, increasing thymic output and partially compensating for the depletion of naïve T cells, as well as countering T cell exhaustion. Therefore, this thymic factor could be a useful therapeutic tool positively contributing to the transient restoration of the immune environment to potentiate immunity and to improve cancer immunotherapy in certain older patients (Figure 1). Further research should be done in order to explore the effects of long-term administration, the optimal therapeutic schedule and the combinations of Biomodulina T with other anti-aging interventions.
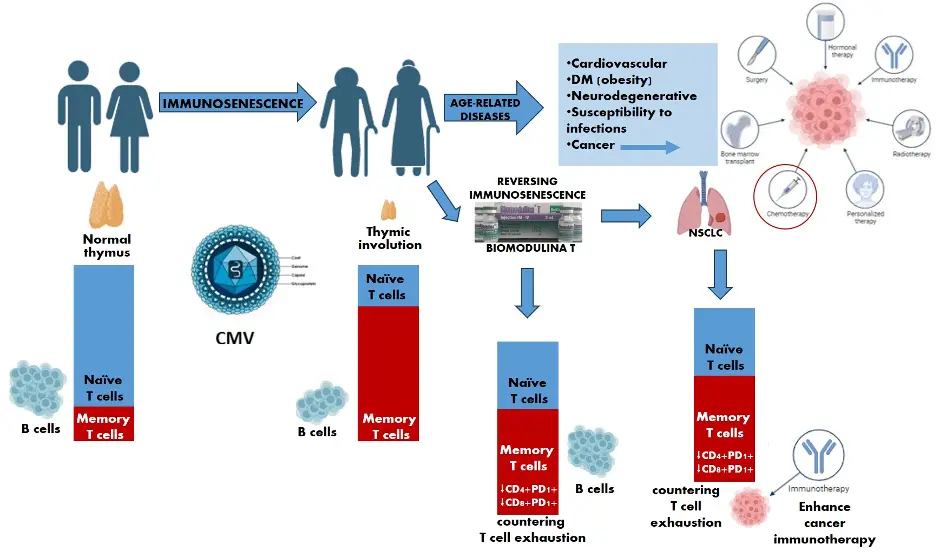
Figure 1. Squematic effect of Biomodulina T on the immune system in older adults and patients diagnosed with advanced lung cancer treated with base-line chemotherapy. Figure created with BioRender.
Acknowledgments
The authors are deeply grateful to the patients and their families, as well as the staff of all institutions involved in the studies referenced in this manuscript, for their invaluable support of this research.
Authors contribution
Saavedra D: Article conceptualization and design, writing-original draft preparation, writing-review and editing.
Añé-Kourí AL, Ledón N, Crombet T, Lage A: Writing-review and editing.
All authors reviewed the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
None.
Copyright
© The Author(s) 2024.
References
-
1. Suarez-Formigo GM, Saavedra-Hernandez D. Biomodulina T may restore immunity in older adults. Medicc Rew. 2020;22(3):54-56.[DOI]
-
2. Saavedra D, Ane-Kouri AL, Barzilai N, Caruso C, Cho KH, Fontana L, et al. Aging and chronic inflammation: highlights from a multidisciplinary workshop. Immun Ageing. 2023;20(1):25.[DOI]
-
3. Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fülöp T, et al. The conundrum of human immune system “senescence”. Mech Ageing Dev. 2020;192:111357.[DOI]
-
4. Fulop T, Larbi A, Dupuis G, Le Page A, Frost , EH , Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960.[DOI]
-
5. Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, et al. Immunology of aging: the birth of inflammaging. Clin Rev Allerg Immu. 2021;64:109-122.[DOI]
-
6. Akbar AN, Henson SM, Lanna A. Senescence of T Lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37(12):866-876.[DOI]
-
7. Kocarnik JM, Compton K, Dean FE, Fu WJ, Gaw BL, Harvey JD, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8(3):420-444.[DOI]
-
8. Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G. Aging, immunity, and cancer. Discov Med. 2011;11(61):537-550.[PubMed]
-
9. Saavedra D, Garcia B, Lorenzo-Luaces P, González A, Popa X, Fuentes KP, et al. Biomarkers related to immunosenescence: relationships with therapy and survival in lung cancer patients. Cancer Immunol Immunother. 2016;65(1):37-45.[DOI]
-
10. Lang PO, Govind S, Aspinall R. Reversing T cell immunosenescence: why, who, and how. Age. 2013;35(3):609-620.[DOI]
-
11. Cardinale A, De Luca CD, Locatelli F, Velardi E. Thymic function and T-Cell receptor repertoire diversity: implications for patient response to checkpoint blockade immunotherapy. Front Immunol. 2021;12:752042.[DOI]
-
12. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14(4):497-510.[DOI]
-
13. Saavedra D, Fuertes SA, Suarez GM, Fuertes SA, Suarez GM, Gonzalez A, et al. Biomodulina T partially restores immunosenescent CD4 and CD8 T cell compartments in the elderly. Exp Gerontol. 2019;124:110633.[DOI]
-
14. Suarez GM, Catala M, Pena Y, Portela S, Añé-Kouri AL, Gonzalez A, et al. Thymic polypeptide fraction Biomodulina T decreases exhausted and terminally differentiated EMRA T cells in advanced lung cancer patients treated with platinum-based chemotherapy. Front Oncol. 2022;12:823287.[DOI]
-
15. Caruso C, Ligotti ME, Accardi G, Candore G. An immunologist’s guide to immunosenescence and its treatment. Expert Rev Clin Immu. 2022;18(9):961-981.[DOI]
-
16. Fulop T, Dupuis G, Witkowski JM, Larbi A. The role of immunosenescence in the development of age-related diseases. Rev Invest Clin. 2016;68(2):84-91.[PubMed]
-
17. Libri V, Azevedo RI, Jackson SE, Mitri DD, Lachmann R, Fuhrmann S, et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132(3):326-339.[DOI]
-
18. Pawelec G. Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immun Ageing. 2012;9(1):15.[DOI]
-
19. Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, Dupuis G, et al. Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr. 2015;41:26-40.[DOI]
-
20. Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47-56.[DOI]
-
21. Frasca D, Diaz A, Romero M, Garcia D, Blomberg BB. B cell immunosenescence. Annu Rev Cell Dev Biol. 2020;36:551-574.[DOI]
-
22. Pawelec G. Immunosenescence and cancer. Biogerontology. 2017;18(4):717-721.[DOI]
-
23. Wolf E, Milazzo S, Boehm K, Zwahlen M, Horneber M. Thymic peptides for treatment of cancer patients. Cochrane Db Syst Rev. 2011;2011(2):CD003993.[DOI]
-
24. Costanzi JJ, Gagliano RG, Delaney F, Harris N, Thurman GB, Sakai H, et al. The effect of thymosin on patients with disseminated malignancies. A phase I study. Cancer. 1977;40(1):14-19.[DOI]
-
25. Goldstein AL. History of the discovery of the thymosins. Ann Ny Acad Sci. 2007;1112:1-13.[DOI]
-
26. Rodriguez Martin RR, Gonzalez Gonzalez O, Rodriguez Gonzalez C, Rodriguez Gonzalez RR. History of the discovery and development of Biomodulina T (InmunyVital®), a useful immunomodulator with a broad range of clinical applications. Int Immunopharmacol. 2023;119:110167.[DOI]
-
27. CECMED-(Center for State Control of Medications EaMD) [Internet]. Havana: CECMED. [cited 2024 Feb 18]. Available from: https://www.cecmed.cu/registro/rcp/biologicos/biomodulinar-t-fraccion-timica
-
28. Orihuela MG, Martínez RS, Gonzalez IC, Paz DS, Orta IA. Efecto terapéutico de la Biomodulina T homeopática con pacientes portadores de enfermedad pulmonar obstructiva crónica. Rev Habanera Cienc Med. 2011;10(3):287-295. Available from: https://revhabanera.sld.cu/index.php/rhab/article/view/1839/1636
-
29. López LC, Rodríguez Marin RR, Perez JR, Lafargue MS, del Sol JMR, Ross EG. Efecto de la Biomodulina T 1000 sobre el timo en ninos con infecciones recurrentes. Rev Cubana Pediatr. 2000;72(1):3-9. Available from: https://www.semanticscholar.org/paper/Efecto-de-la-biomodulina-T-1000-sobre-el-timo-en-L%C3%B3pez-Mar%C3%ADn/b8a8e09d058ea98e2d50fd9300ac05bf3d66590b
-
30. Morales LAG, Rodriguez RFL, Martin RR, Gonzalez-Quevedo A, Carriera RF, Silva NM. Estudio Fase II de tratamiento de pacientes con Esclerosis multiple exacerbante-remitente con Biomodulina T. Rev Mex Neuroci. 2007;8(1):28-31. Available from: https://previous.revmexneurociencia.com/wp-content/uploads/2014/06/Nm071-06.pdf
-
31. Canete R, Afonso JA, Brito K. The effects of the uncontrolled use of Biomodulina T on the severity of severe acute respiratory syndrome-coronavirus 2 infection in older Cuban adults: an open label evaluation. Curr Ther Res. 2022;96:100662.[DOI]
-
32. Hernandez IC, Suarez VM, Ramos EH, Marrero YT, Dominguez GD, Perez YD, et al. BIOMODULINA T® modulates lymphocyte compartments in institutionalized Cuban geriatric patients. J Cell Immunol. 2022;4(2):79-91.[DOI]
-
33. Ramos EH, Suárez VM, Hernández IC, Gomez RP, Rivera DG, Zamora MCR, et al. Effect of Biomodulina-T® and VA-MENGOC-BC® on lymphocyte subpopulations in older adults. Exp Gerontol. 2021;153:111497.[DOI]
-
34. Majumdar S, Nandi D. Thymic atrophy: experimental studies and therapeutic interventions. Scand J Immunol. 2018;87(1):4-14.[DOI]
-
35. Fülöp T, Larbi A, Hirokawa K, Mocchegiani E, Lesourd B, Castle S, et al. Immunosupportive therapies in aging. Clin Interv Aging. 2007;2(1):33-54.[DOI]
-
36. Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205(7):1701-1714.[DOI]
-
37. Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120(24):4882-4891.[DOI]
-
38. Chen IH, Lai YL, Wu CL, Chang YF, Chu CC, Tsai IF, et al. Immune impairment in patients with terminal cancers: influence of cancer treatments and cytomegalovirus infection. Cancer Immunol Immunother. 2010;59(2):323-334.[DOI]
-
39. Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21(5):2772-91.[DOI]
-
40. Suarez GM, Ane -Kouri AL, Gonzalez A, Lorenzo-Luaces P, Neninger E, Salomon EE, et al. Associations among cytokines, EGF and lymphocyte subpopulations in patients diagnosed with advanced lung cancer. Cancer Immunol Immunother. 2021;70(6):1735-1743.[DOI]
-
41. Bailur JK, Pawelec G, Hatse S, Brouwers B, Smeets A, Neven P, et al. Immune profiles of elderly breast cancer patients are altered by chemotherapy and relate to clinical frailty. Breast Cancer Res. 2017;19(1):20.[DOI]
-
42. Sun DP, Wang L, Ding CY, Liang JH, Zhu HY, Wu YJ, et al. Investigating factors associated with thymic regeneration after chemotherapy in patients with Lymphoma. Front Immunol. 2016;7:654.[DOI]
-
43. Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur J Clin Invest. 2005;35(6):380-387.[DOI]
-
44. Saavedra D, Crombet T. CIMAvax-EGF: A new therapeutic vaccine for advanced non-small cell lung cancer patients. Front Immunol. 2017;8:269.[DOI]
-
45. Rodriguez PC, Popa X, Martínez O, Mendoza S, Santiesteban E, Crespo T, et al. A Phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22(15):3782-3790.[DOI]
Copyright
© The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




