Abstract
The outcomes of older adults with cancer are still dismal despite some progress within the last years. This is mainly due to comorbidities, overall frailty, and differences in disease biology. The better understanding of tumor biology and immunology has enabled the use of targeted therapies and immunotherapies that are potentially better tolerated than traditional chemotherapies. Several randomized trials were recently published that demonstrated a benefit of integrated onco-geriatric care including geriatric interventions on treatment-related toxicities and quality of life during cancer treatment of older adults. Furthermore, other pivotal trials adapted treatment intensities based on frailty. Despite these efforts, older and frail adults are still underrepresented in clinical trials. This leads to a major lack of an evidence-based standard of care in geriatric oncology. In this narrative review, we discuss pivotal trials, practical implications, under- and overtreatment, altered pharmacokinetics at older age, and future perspectives for geriatric oncology.
Keywords
1. Introduction
The care for older adults with cancer often brings additional challenges, compared to younger patients and outcomes are usually inferior. This is mostly due to comorbidities, general frailty[1], and probably differences in disease biology[2]. Much progress has been made in prediction of treatment-related toxicities und assessment of vulnerabilities within in the last two decades[3]. This enabled the introduction of geriatric interventions into cancer treatment concepts and the first seminal works on active modification of treatment intensities based on vulnerabilities[4-6]. Furthermore, several targeted anti-neoplastic drugs were introduced within the last years, which are potentially better tolerated than traditional chemotherapies. Despite this, we are still far from an evidence-based standard of care in geriatric oncology, particularly in the case of very frail patients. As we learned to implement disease-specific characteristics to tailor targeted therapies, we probably need to learn about adjustments in accordance to other personalized factors, such as specific comorbidities, or body composition. In this narrative review, we summarize key concepts of toxicity prediction, impact of geriatric impairments, integration of geriatric interventions, altered pharmacokinetics at older age, immune-senescence, and key studies in geriatric oncology with a specific focus on potential future research questions.
2. Detection of Vulnerabilities and Their Impact on Treatment Tolerability
In this section, we summarize tools that predict treatment-related toxicities based on geriatric impairments, and trials that aimed at reducing toxicities by geriatric interventions. For the purpose of this review, we define geriatric assessment (GA) as the evaluation of geriatric impairments, and comprehensive geriatric assessment (CGA) as GA followed by initiation of geriatric interventions, based on the diagnosed deficits.
2.1 Geriatric screening tools
A GA is usually considered as time-consuming. Not all older adults have geriatric impairments. Thus, the use of geriatric screening (GS) tools is recommended by the American Society of Oncology[7], the National Comprehensive Cancer Network[8], and the International Society of Geriatric Oncology[9] for selection of patients who potentially benefit from a comprehensive geriatric assessment and those, on the other hand, that are unlikely to have further deficits. The most commonly used tools are the Geriatric 8 (G8) and vulnerable elderly survey-13 (VES-13) questionnaires[10]. The G8 was developed specifically for cancer patients and contains eight questions on nutrition, weight loss, body mass index, mobility, psychosocial issues, self-rated health, age, and polypharmacy[11]. The completion of the questionnaire takes less than five minutes and a score of ≤ 14/17 points indicates a high risk for geriatric impairments[10]. The G8 was demonstrated to be associated with functional decline[12], chemotherapy-associated toxicities[13], and overall survival[12]. Recently, a G8 score ≤ 14 was used as one of the inclusion criteria within the Effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer (GERICO) trial[14], providing now new evidence from a randomized phase 3 trial for the sequential approach to perform a CGA based on a GS.
The VES-13 was validated for prediction of functional decline in older adults within the community setting, set with a score of ≥ 3[15]. In cancer patients, VES-13 can predict chemotherapy-associated toxicities[12]. In comparison to G8, VES-13 has a stronger focus on functional capacities. Thus, both tools together increase sensitivity and specificity[16].
The use of screening tools gives valuable information but does not replace an appropriate assessment of relevant comorbidities and a CGA as geriatric interventions cannot be initiated based on GS.
2.2 Prediction tools for chemotherapy-related toxicities
Two easy-to-use tools for prediction of chemotherapy-related toxicities are available, namely the Cancer and Aging Research Group (CARG) and the Chemotherapy Risk Assessment Scale for High Age (CRASH) score. The CARG score was validated to estimate the occurrence of any chemotherapy-related toxicity Common Terminology Criteria for Adverse Events (CTCAE) III-V utropenia CTCAE ≥ III° occurred in 30% of HMA/VEN group compared to 10% in cancer patients ≥ 65 years. This score includes assessment of age, cancer entity, mono- versus polychemotherapy, planned dose reductions for the first cycle, hemoglobin, creatinine clearance, hearing impairments, falls, required assistance with medication intake, walking ability, and social activities[17,18].
The CRASH score was validated in cancer patients ≥ 70 years to predict CTCAE III-IV° non-hematological and CTCAE IV° hematological toxicities. It includes instrumental activities of daily living (IADL), ECOG performance status, Mini Mental State Examination (MMSE), Mini Nutritional Assessment (MNA), diastolic blood pressure, elevated lactate dehydrogenase, and intensity of intended chemotherapy[19].
Both scores were developed before the introduction of immune-checkpoint inhibitors and the frequent use of small molecule targeted therapies. Data on their predictive ability in patients receiving those therapies are lacking.
Although both scores are widely used to aid clinical decision making on treatment intensity[20], studies that uses their prediction to guide treatment decisions on different chemotherapy intensities are scarce and not completed yet. With great expectations, results of the DURATION trial (NCT03345810) can be awaited: In this randomised phase 2 trial, older patients with non-small cell lung cancer are allocated to receive mono- or doublet chemotherapy based on their CARG score, followed by either four cycles or two cycles of chemotherapy and subsequent treatment with the programmed death-ligand 1 (PD-L1) inhibitor durvalumab. As primary endpoint, treatment-related toxicities are assessed[21].
Prediction of chemotherapy-related toxicities and GS tools share many similar aspects but cannot be replaced by each other. To avoid patient fatigue and inefficient use of health-care resources, we suggest to use GS tools with the clear aim to select patients who likely profit from a CGA and tools for prediction of chemotherapy-related toxicities in case a conventional chemotherapy is planned (e.g., for discussion of potential higher-grade toxicities during shared decision making).
2.3 Comprehensive geriatric assessment and its impact on treatment-related toxicities
Several clinical trials included a GA to characterize the patient population and/or for prognostication[22,23]. Despite this, the impact of geriatric interventions as indicated by a GA were only recently evaluated: Six randomized clinical trials on implementation of such a CGA into treatment of geriatric cancer patients have been published within the last three years. Of those, four reached their primary endpoint. As these trials have been practice-changing, they are summarized in Table 1 and a selection is further described here in greater detail. Although the trials assessed mostly the same standard domains of a GA, such as functional status, cognition, nutrition, comorbidities, psychosocial status, and quality of life, instruments of the assessments differed depending on established local structures and preferences (Table 1).
| Study | GAP-70[4] | GAIN[5] | GERICO[14] | NCT02359838[24] | INTEGERATE[6] | 5C[25] |
| Design | Cluster-randomized trial, multi-center | RCT, single-center | RCT, bicentric | RCT, single center | RCT, multicentre, open-label | RCT, multicenter, single-blind |
| Patients (age, cancer entities, intended treatment) | ≥ 70 y, lymphoma/solid cancer with palliative treatment intent, ≥ 1 geriatric deficit | ≥ 65 y, solid cancer (all stages) | ≥ 70 y, adjuvant or first-line palliative chemotherapy for CRC, G8 ≤ 14 | ≥ 75 y, hematological neoplasia, first visit at center, transplant-ineligible, treatment intended | ≥ 70 y, solid cancer or DLBCL, planned for chemotherapy, targeted therapy, or immunotherapy | ≥ 70 y, solid cancer, myeloma or DLBCL, planned for first-/second-line chemotherapy, immunotherapy or targeted therapy, ECOG 0-2 |
| Intervention(s) | IA: oncologist received recommendations for GIN SOCA: standard | IA: multidisciplinary CGA was included into treatment concept SOCA: GA results sent to oncologist for consideration | IA: CGA-based GIN SOCA: standard care without GA | IA: geriatric consultation, GIN as indicated SOCA: standard care without CGA | IA: CGA integrated into oncology care, including GIN SOCA: standard care without GA | IA: CGA integrated into oncology care SOCA: standard care without GA |
| Primary endpoint | TRT CTCAE ≥ 3° | TRT CTCAE ≥ 3° | Chemotherapy completion without dose reductions or delays | One-year OS | Changes in QoL over 24 weeks, assessed using ELFI | Changes in QoL assessed by EORTC-QLQ-C30 |
| Primary outcome | Relative risk reduction for TRT=0.74 (95% CI, 0.64-0.84, P = 0.0001) | Absolute reduction of TRT=10.1% in GAIN vs. SOCA (95% CI, -1.5 to -18.2%; P = 0.02) | Higher chemotherapy completion rates in IA: 45% vs. SOCA: 28%, P = 0.0366 | No significant difference in OS (difference: 2.9%, 95% CI, -9.5% to 15.2%, P = 0.65) | Better ELFI change scores (largest effect at 18 weeks: mean difference in change 9.8; 95% CI, 2.4-17.2, P = 0.010, corrected P = 0.030, effect size = 0.48) | Global QOL of 4.4 points (95% CI, 0.9-8.0) favoring SOCA, primary endpoint failed |
| Primary endpoint reached? | YES | YES | YES | NO | YES | NO |
| Secondary endpoints | Dose reductions in c1, RDI (3 months), 6m-/ 12m-OS, risk of falls | Advance directive completion, health care use, chemotherapy dose modifications | TRT, OS, QoL | Unplanned health care utilization within 6 months, documented end-of-life goals-of-care discussions | Unplanned hospital admissions, OS | Functional status, toxicities CTCAE III-V°, health care use; cancer treatment plan modification; OS |
| Secondary outcomes | IA: more dose reduction at c1, lower RDI, equal 6m-/12m-OS, lower risk for falls | Absolute increase in advance directive completion (GAIN: 28.4% vs. SOCA: 13.3%, P < 0 .001; no significant differences in health care utilization, chemotherapy dose modifications, and OS | Less toxicities in IA (28% vs. 39% in SOCA, P = 0.156), decreased burden of illness (P = 0.048) and improved mobility (P = 0.008) in IA, no difference in OS | No difference in health care utilization; significantly more end-of-life discussion (OR = 3.12, 95% CI, 1.03-9.41) | No difference in OS; fewer unplanned hospital admissions at 24 weeks in IA (multivariable-adjusted incidence rate ratio 0.60 [95% CI 0.42–0.87]; P = 0.0066) | No difference in OS, change in treatment plan, unplanned hospitalization/emergency department visits, and treatment toxicity between groups |
| Comments | Effect of CGA mainly in patients with G8 score ≤ 11 (OR 3.76, 95% CI, 1.19-13.45) | Patients with indolent and aggressive neoplasia included; very few received newer agents | GA was mostly performed on day of treatment start, thus, treatment plans were rarely changed as a result of the GA. > 50% of participants were treated with curative intent and ~1/3 had a G8 score >15 points. Thus, the trial might have missed the population who benefits most | |||
| Included elements of the geriatric assessment | ||||||
| Functional status | ADL, IADL, History of falls, OARS Physical Health, TUG, SPPB | KPS, TUG, IADL, MOS physical functioning, Number of falls in last 6 months | ADL, IADL, Gait speed 10 m, Handgrip assessed with the Jamar Dynamometer | IIP | ECOG, KPS, ADL, IADL, TUG | ADL, IADL, Grip strength, SPPB, self-reported falls, ECOG |
| Cognition | Blessed OMC,Mini-COG | Blessed Orientation-Memory-Concentration | MMSE | IIP | - | Mini-COG |
| Nutrition | BMI, MNA, reported weigh loss | BMI, percent unintentional weight loss | MNA | IIP | MNA | Weight and appetite, serum albumin level |
| Comorbidities | OARS Comorbidity | OARS Physical Health Sub-scale | Medicalrecord review | IIP | CCI | CCI |
| Psychological Status | GAD-7, GDS | Mental Health Inventory, Depression and Anxiety questions | GDS | IIP | PHQ-9, GAD-6 | PHQ-9 |
| Social Functioning and Support | OARS Medical Social Support | MOS Social Activity Limitation: MOS Social Support Subscale | Unstructured assessment | IIP | Unstructured assessment | Unstructured assessment |
| Spiritual Well-Being | - | Duke Spiritual Scale | - | IIP | - | - |
| QoL | - | FACT-G | EORTC-QLQ- C30, EORTC-QLQ-ELD14 | IIP | EORTC-QLQ-C30, EORTC-QLQ-ELD14 | EORTC-QLQ-C30 |
| Polypharmacy | Unstructured | - | Assessment of medication list based onSTART/STOPP criteria | IIP | Unstructured assessment | Brown bag medication review |
ADL: activities of daily living; BMI: body mass index; CCI: charlson comorbidity index; CI: confidence interval; CRC: colorectal cancer; DLBCL: diffuse large B-cell lymphoma; ECOG: Eastern Cooperative Oncology Group performance status; ELFI: elderly functional index; EORTC-QLQ-C30: European Organisation for the Research and Treatment of Cancer QOL core version 30 items questionnaire; EORTC-QLQ-ELD14: European Organisation for the Research and Treatment of Cancer QOL Elderly 14 Questionnaire; FACT-G: the functional assessment of cancer therapy-general; GAD-6: generalized anxiety disorder scale-6; GDS: geriatric depression scale; G8: geriatric 8 questionnaire; GIN: geriatric intervention; IA: intervention arm; IADL: instrumental activities of daily living; IIP: insufficient information provided; KPS: karnofsky performance status; m: months; MMSI: minimal mental state examination; MNA: minimal nutrition assessment; MOS: medical outcomes study; OR: odds ratio; OS: overall survival; PHQ-9: patient health questionnaire-9; RDI: relative dose reductions; SOC: standard-of-care arm; SPPB: short physical performance battery; TRT: treatment-related toxicities; TUG: timed up and go test.
2.4 The GAIN study
The geriatric assessment–driven intervention (GAIN-) study included patients ≥ 65 years with a variety of solid cancers of all stages starting a new chemotherapy[5]. All patients underwent a GA at randomization. In the intervention arm (GAIN), a multidisciplinary teamC consisting of an oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker and nutritionist, designed an intervention plan and initiated appropriate referrals based on the GA. In the standard-of-care arm (SOC), the treating oncologists only received the GA results for information purposes. Chemotherapy-related toxicities CTCAE III°-V° were significantly reduced in GAIN vs SOC arm (50.5% vs. 60.6%; absolute reduction: 10.1%, 95% CI, -1.5 to -18.2%; P = 0.02). Thus, the study reached its primary endpoint.
2.5 The GAP70+ study
The cluster-randomized GAP-70+ trial[4] included patients ≥ 70 years with lymphoma or metastasized solid cancers and at least one geriatric impairment starting a new chemotherapy.
All patients received a GA. In the intervention arm, oncologists received the GA results together with recommendations for interventions. In the SOC arm, oncologists received GA results without recommendations. The primary endpoint, chemotherapy-related toxicities CTCAE III-IV° were significantly reduced in the intervention arm, compared with SOC (51% vs. 71%, relative risk reduction 0.74; 95% CI, 0.64 to 0.84, P = 0.0001). In addition, frequency of falls and polypharmacy was also reduced in the intervention arm. The overall survival after one year was similar in both groups.
2.6 The NCT02359838 - study
The NCT02359838 - study evaluated a geriatric consultation model for transplant-ineligible patients ≥75 years with hematological malignancies. Patients classified as frail or pre-frail were randomized to receive the standard-of-care treatment with or without a geriatric consultation. Geriatric interventions were initiated after the geriatric consultation as indicated. The primary endpoint, the one-year OS, was not significantly different (difference: 2.9%; 95% CI, -9.5% to 15.2%, P = 0.65). In addition, the frequency of emergency department visits, hospital admissions, or days in hospital were also not significantly different in both arms. A geriatric consultation favoured end-of-life goals-of-care discussions (OR = 3.12, 95% CI, 1.03 to 9.41)[24].
The discussed trials used different models of onco-geriatric co-management, such as integration of a full multidisciplinary team into the oncology service, an externally provided CGA for further use by the oncologist, or a geriatric consultation service that initiated referrals based on GA deficits. Thus, the choice of the onco-geriatric care model can now be adapted to the local situation.
None of the six trials demonstrated a superior overall survival for the respective geriatric intervention arms compared to standard-of-care. This is not surprising as the patient populations included many patients with advanced cancers and were very heterogenous.
4/6 trials (Table 1) assessed treatment-related toxicities as outcome. In three out of these four trials, a significant reduction of treatment-related toxicities was observed. The Canadian 5C trial was the only trial that did not demonstrate such a benefit. Of note, the 5C trial allowed performance of the GA as late as on the day of treatment commencement. Therefore, a change in treatment plans, including dose reductions during the first cycle, was not performed based on the GA results and could explain the failure of this trial. In comparison, in GAP70+ trial, a higher percentage of patients received monotherapies and/or dose reductions of the first treatment cycle based on GA results. In addition, GAP70+ trial included only patients with at least one documented GA deficit, the GERICO trial included patients only with a positive GS (G8 score ≤14) and demonstrated the largest benefit for patients with a G8 score ≤11 points. In the C5 trial, more than one third of patients had a G8 score above 15 points and were most likely not frail. Thus, patients who profit most from interventions might have been underrepresented in this trial, potentially further diluting the effect of the geriatric interventions.
The second trial that did not meet its primary endpoint was the NCT02359838- study. It included a very heterogenous population of indolent and aggressive hematological malignancies. During the recruitment period, several combination therapies, such as hypomethylating agents with venetoclax for treatment of acute myeloid leukemia, or daratumumab in combination with dexamethasone and lenalidomide for multiple myeloma, were not yet available. Because outcomes of aggressive hematological malignancies are predominantly determined by disease control, failure of the primary endpoint 'overall survival' might be difficult to modify by geriatric interventions if the disease itself is not controlled. In addition, only 80% of patients within the intervention arm received a geriatric consultation as intended. This might have further biased the results.
In conclusion, based on these randomized controlled trials, the onco-geriatric approach is backed by strong evidence and should be included in the routine cancer care of older adults as well as in clinical trials. Within the next years, it will be a major challenge to integrate CGAs consequently into routine clinical care, to generate adequate human resources to perform this additional effort, and to ensure reimbursement policies.
3. Impact of Distinct Impaired GA Domains: Sarcopenia
Distinct impairments of single GA domains might predict specific toxicities and vulnerabilities. Here, we focus on sarcopenia as it has several implications for geriatric interventions, as well as in its consequence as metabolic disturbance. According to the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), sarcopenia is defined as low muscle strength combined with low muscle quantity or quality. It is classified as severe if physical performance is impaired in addition[26]. Sarcopenia is related to many unfavourable health outcomes, such as an increased risk of falls, loss of independence, and death[26]. In cancer patients, sarcopenia can exist as part of cancer cachexia, or independently, and has been described as adverse factor leading to increased treatment-related toxicities, or surgery-associated complications[27].
In clinical practice, the 5-item SARC-F (strength, assistance walking, rise from a chair, climb stairs, and falls) questionnaire is recommended as screening tool to exclude people at high risk for adverse sarcopenia-related functional outcomes[26,28]. In addition to the patient-rated tool, a proxy-reported version of the questionnaire is now available in case the patient cannot answer the questions due to acute severe illness[29]. For diagnosis of sarcopenia, at least the measurement of muscle strength by hand grip strength and/or chair rise test and assessment of muscle mass by magnetic resonance imaging (MRI) or computed tomography as gold standards are recommended[26]. Alternatives for the quantification of muscle mass are dual-energy X-ray absorptiometry, or bioelectrical impedance analysis[26]. Once the diagnosis is set, treatment options besides physical exercise[30,31] are limited and controversially discussed[31]. In the oncological setting, a diagnosis of sarcopenia gives valuable information on putative treatment tolerability[32-35] and carries the potential for prehabilitation[36-38].
Muscles are nowadays increasingly recognized as a metabolic compartment[39]. Thus, the impact of sarcopenia on health-related outcomes might have further impact besides its role in physical impairments: Differences in body composition might interfere with pharmacokinetic parameters, such as distribution, metabolism, and clearance of several drugs. Most chemotherapies are dosed based on body surface area (BSA) due to historical reason rather than solid evidence. Firstly, BSA poorly correlates with pharmacokinetic parameters of cytotoxic drugs[40]. Secondly, dosing based on BSA reduces inter-patient variability in only 15% of those drugs[41] which is reviewed in[42]. Besides the growing body of evidence indicating that sarcopenia predisposes to increased treatment-related toxicities, only few data on modelling of pharmacokinetics in dependence of muscle mass exist[43,44]. Especially in vulnerable older adults, dosing of chemotherapies based on body composition/sarcopenia could provide an innovative approach[45] and should be further evaluated in clinical trials.
4. Pharmacological Changes in Older Adults with Potential Impact on Treatment Tolerability
Pharmacokinetics in older adults can vary significantly, when compared with younger adults due to organ aging, decreased gastric mobility and splanchnic blood flow, or body composition (as described above)[42]. Further, cachexia- or frailty-related hypalbuminemia can influence clearance, peak concentrations, and metabolism of mainly protein-bound substances; changes in cytochrome p450 function can also impact drug metabolism[42]. The impact of these factors on treatment tolerability and efficacy are poorly understood and rarely assessed in clinical trials. As pharmacokinetic measures often require extensive effort and frequent (blood/urine) sample collections, its assessment might be hampered especially in frail patients. Despite that, implementation of personalized pharmacokinetics into drug dosing could potentially reduce toxicity and increase efficacy; thereby, studies that assess these questions are highly desired.
5. Less Can be More: Practice-changing Trials for Frailty-adapted Changes in Treatment Intensity
In the GAP70+ trial, which is described in greater detail above, the likelihood to receive the first cycle of chemotherapy at a reduced dose was significantly higher in the intervention arm (adjusted relative risk (RR) =1.38; 95% CI, 1.06-1.78, P = 0.015). This continued and more patients received monotherapies than combination therapies (interventions arm vs. standard arm: 23% vs. 18%) which resulted in an overall reduced dose intensity without impact on overall survival. In conclusion, this suggests that the balance between treatment efficacy and tolerability is one of the key factors in frail patients.
A key example for the profound effect of dose adjustments is the large phase 3 GO2 trial. This trial evaluated the non-inferiority of dose adjustments in patients with advanced gastroesophageal cancer who were considered as unfit to receive standard triplet therapy (epirubicine, oxaliplatin, and capecitabine, EOC) by their treating physician[46]. GA was performed before randomization although the results were not further communicated to the treating oncologist. Notably, even the 100% dose arm represented a dose modification from the treatment standard, consisting of only two of the three standard drugs (EOC). Patients were randomized to receive 60%, 80%, or 100% dose of oxaliplatin and capecitabine. Patients were offered a second randomization between 60% dose intensity compared with best supportive care if the latter was considered in general. The median age was 76 years (range: 56-96 years) and 58% were defined as severely frail. Non-inferiority was set as less than 34 days reduction in progression-free survival (PFS). Of note, this boundary was chosen by a forum of physicians together with patients. The key findings were as follows:
• Dose reductions were not related to an inferior PFS (80% vs. 100%: HR (PFS) = 1.09 [95% CI, 0.89-1.34]; 60% vs. 100%: HR (PFS) = 1.10 [95% CI, 0.90-1.34]).
• Overall treatment utility was non-significantly better for the lowest dose intensity (60%), even in younger and less frail patients without loss of disease response (100% vs. 60%: HR = 0.63 [90% CI, 0.36-1.11])[46].
Thus, the GO2 trial demonstrated again that a more tentative treatment approach can compensate for frailty without loss of disease control.
Besides its importance, the GO2 trial has some caveats: Patients were included if they were deemed unfit for triplet therapy based on the clinical judgment of their treating oncologist. In comparison, the GERICO trial included patients based on a positive geriatric screening (G8 score ≤ 14)[47]. Despite the important role of clinical judgment that should not be underestimated, the trial inclusion was less well-defined impeding its transfer to different settings.
6. Less is Not Always More: Lessons Learnt from Randomized Trials
Especially the intensive treatment approaches for aggressive hematological malignancies are often hardly tolerated by older adults due to the high treatment-associated morbidity and mortality[1,48-50]. For many diseases, such as acute myeloid leukemia (AML), this led to a rigorous down-scaling of treatment intensity with improved treatment tolerability at the expense of a worse disease control. Among these options for AML are low-dose cytarabine containing regimens[51] or hypomethylating agents[52]. With the advent of several targeted drugs, this treatment paradigm was recently challenged. In this section, we will discuss three scenarios in which intensified treatment approaches are not only beneficial for disease control but several patient-reported outcomes.
In the treatment of AML in older adults, hypomethylating agents (HMAs) were demonstrated to have similar outcomes as intensive induction chemotherapy: In a large pooled European registry data analysis that included > 3,000 patients above the age of 70 years, no significant difference in median overall survival (mOS) was demonstrated between the treatment groups[53], but patients who survived the first four months had a better long-term survival when receiving an intensive chemotherapy[53]. In addition, the use of HMAs was further justified by data that demonstrated more time spend home when being treated with HMAs in comparison to intensive chemotherapy approaches[54]. Despite this, the mOS with HMAs as monotherapy is usually less than one year[52]. This situation has changed with the addition of the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax: The combination of HMAs with venetoclax (VEN) showed a superior mOS in comparison to HMAs alone in the randomized phase 3 "VIALE-A" trial (14.7 months for HMA/VEN and 9.6 months for HMA alone, HR for death, 0.66; 95% CI, 0.52 to 0.85; P < 0.001)[55]. As expected, adverse events were more frequent in the HMA/VEN arm than with HMA alone, e.g. febrile neutropenia CTCAE ≥ III occurred in 30% of HMA/VEN group compared to 10% in the HMA alone group[55]. Despite the higher treatment intensity and the frequent adverse events, a combined analysis of the VIALE-A and VIALE-C trial, that evaluated low-dose cytarabine in combination with VEN, revealed a longer time to deterioration in quality of life (QoL) as assessed with EORTC QLQ-C30 global health status (GHS/QoL) and physical functioning (PF), PROMIS Cancer Fatigue Short Form 7a (Fatigue), and EQ-5D-5L health status visual analogue scale (HS-VAS) as compared with HMA or LDAC monotherapy[56].
The AGILE trial, another important phase 3 trial in older patients with AML, evaluated the combination of HMAs with the isocitrate dehydrogenase (IDH-)-1 -inhibitor ivosidenib (IVO) in comparison to HMAs alone. Again, a superiority with regard to mOS could be demonstrated for the combination of HMA/IVO (mOS 24.0 months; hazard ratio for death, 0.44; 95% CI, 0.27 to 0.73; P = 0.001) in comparison to HMA alone (mOS 7.9 months)[57]. In addition, QoL assessment also favored AZA/IVO over AZA monotherapy in all subscales of EORTC QLQ-C30 questionnaire[57].
Another change in treatment paradigms is the intensification of frontline treatment in older patients with multiple myeloma ineligible for autologous stem-cell transplantation. The addition of daratumumab to lenalidomide and dexamethasone (D-Rd) in comparison to lenalidomide and dexamethasone (Rd) alone in the MAIA trial improved the progression-free survival (PFS) at 30 months (70.6 (D-Rd) vs. 55.6% (Rd); HR for disease progression or death, 0.56; 95% CI, 0.43 to 0.73; P < 0.001)[58]. A subgroup analysis of this trial compared frail patients as defined by age, Charlson comorbidity index, and baseline ECOG score, with non-frail patients. It revealed a better PFS in non-frail patients in comparison to frail patients, but the benefit of daratumumab addition was maintained in the subgroup of frail patients (PFS not reached (D-Rd) vs. 30.4 months (Rd); HR, 0.62; 95% CI, 0.45-0.85; P = 0.003)[59]. Only the subgroup of frail patients with International Staging System (ISS) stage III disease did not benefit from daratumumab addition. Of note, although treatment discontinuation rates were highest in the frail subgroup, which likely explains the inferior PFS in comparison to non-frail patients, discontinuation rates were lower in the D-Rd arm in comparison to Rd. This further demonstrates that a treatment intensification is not related to worse tolerability per se. Qol was assessed by EORTC QLQ-C30 questionnaire and underlined the benefit of daratumumab addition as scores were consistently higher in the D-Rd group independently of age or ECOG scores. The same benefit was observed for the pain reduction as early as cycle 3 (P = 0.0007)[60].
These examples clearly demonstrate that a treatment intensification, especially if this leads to an improved disease control, can indeed provide survival benefits without a negative impact on QoL. Risks and benefits should be balanced with great caution depending on patient's status, geriatric impairments, the type and biology of the underlying cancer diagnosis, and treatment options.
7. Just Another Way? Cancer Immunotherapies in Older Adults
The introduction of therapies that modify the hosts' responses against tumors, such as immune - checkpoint inhibitors (ICI) or chimeric antigen receptor T cell therapy (CART), have added another promising treatment principle into cancer therapies. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) ICI have been shown to be well-tolerated in older adults, while retaining clinical benefit in various solid cancers[61] as well as Hodgkin's lymphoma[62]. Other checkpoint inhibitors such as lymphocyte-activation gene 3 (LAG-3) inhibitors are emerging and seem to be equally efficacious and tolerable in older adults in the setting of advanced cutaneous melanoma[63]. Several bispecific antibodies hold the promise of substituting potentially more toxic chemotherapeutics and are under evaluation[64], while others such as mosunetuzumab, or blinatumomab have already been approved in follicular lymphoma and B acute lymphoblastic leukaemia (B-ALL), respectively. Antibody-drug conjugates could potentially reduce toxicity through targeted drug delivery[65]. Several agents are already established in the relapse setting (enfortumab vedotin – urothelial cancer, inotuzumab-ozogamicin-B-ALL, and trastuzumab deruxtecan – breast cancer) while others have already found their way into first line treatment (brentuximab vedotin - Hodgkin's lymphoma, polatuzumab vedotin-DLBCL)[65]. Additionally, in the last few years, the immunologic armamentarium in both immature and mature B cell malignancies has been revolutionized with the use of CART. Real world evidence from older adults with DLBCL treated with CART demonstrate that older adults have at least equivalent outcomes as younger patients[66,67]. For patients with multiple myeloma, CAR-T-cell therapies show also promising results[68,69]. In the phase 2 KarMMa study that led to the FDA approval of idecabtagene vicleucel (ide-cel, ABECMA), 35% were ≥ age 65 and showed similar response and toxicity rates[70,71]. Of note, in a recent analysis of 4522 patients within the Flatiron Health Electronic Health Record - derived data base revealed a significantly reduced probability to fulfil the eligibility for CAR-T-cell therapy according to the FDA approval for patients ≥ 70 years[72]. Thus, the excellent results with CAR-T-cell therapies in older adults might be partially biased by patient selection.
As immune-senescence including, inter alia, T cell anergy, exhaustion, and senescence, is closely related to biological aging[73], the question arises whether this affects the treatment efficacy of immunotherapies. Prediction of ICI efficacy is still at an early stage[74] but many features related to immune-senescence are of potential impact[73]. For example, T cell senescence was demonstrated to correlate with inferior treatment response towards ICI in a small pilot trial of melanoma patients[75] which is in line with recent data in advanced NSCLC[76].
Another phenomenon with potential impact on immunotherapies is clonal hematopoiesis of indeterminate potential (CHIP) which refers to the presence of a hematopoietic clone with a common leukemia driver mutation without an underlying hematopoietic disease[77]. As CHIP results in a proinflammatory state, an influence of CHIP-positive tumor-infiltrating lymphocytes on the tumor microenvironment is reasonable. Specific CHIP-mutations, such as mutations in DNA-damage-response genes like TP53, confer to a survival benefit[78]. Such a positive selection bias could potentially impact survival of CART cells, but no solid data on these questions are available so far.
Advances in this field may improve our understanding of immune therapies and inform treatment decisions in future.
8. Underrepresentation of Older Adults in Clinical Trials
Clinical trials that establish the SOC for the treatment of cancer often exclude older adults based on their age, or comorbidities[79]. Although real-world data can provide valuable evidence, the ultimate goal to improve evidence-based medicine in the field of geriatric oncology is to provide evidence from randomized controlled trials. In a recent survey among National Cancer Institute (NCI) Community Oncology Research Program members, comorbidity-attributed trial ineligibility, concern about potential toxicities, decline of patients or family caregivers to participate, and the general lack of trials that are relevant to older patients were identified as potential barriers towards trial inclusion for older adults[80]. Based thereon, the NCI has published recommendations on trial design for older adults with cancer[81]. The strict incorporation of GA elements was recommended. Furthermore, several strategies to facilitate enrolment of older adults into clinical trials were suggested. For randomized trials, the SOC arm might be treated with other treatment intensities as in younger adults; in such cases, the design of a trial that includes only older adults might be superior. The inclusion of a parallel cohort of only older adults can be useful to evaluate safety and feasibility and allows for a separate design in comparison to the regular study design. Another approach is a stratified randomization approach, e.g., a stratification for age and fitness to minimize imbalances of crucial factors between study arms. Moreover, broad eligibility criteria, the involvement of community hospitals, and intervention delivery or follow-up requirements that do not differ fundamentally from routine patient care were recommended[81].
9. Conclusions: Expectations and Demands for Future Trials in Geriatric Oncology
Much progress has been made in prediction of toxicities and reduction of those by geriatric interventions in older adults. Despite this, treatment tolerability and outcomes remain inferior compared to younger patients. Selected trials, as described above, have started to tailor treatment intensity and chemotherapy dosing based on toxicity prediction and geriatric assessments.
Personalized treatment approaches based on mutational profiling were established in the last decade for several cancer entities, such as non-small lung cancer or colorectal cancer which has revolutionized cancer therapy. In addition, the impact of the host is increasingly recognized as important, especially for immunotherapies. Deciphering the impact of early use of targeted therapies and immunotherapies in older adults is highly desired.
In conclusion, the importance of "staging the aging" is widely recognized; despite that, the integration of translational aspects of aging and cancer biology into treatment and study concepts for older patients in addition to functional capacities is currently underrepresented. We probably need to recognize and integrate multiple layers of personalized therapy modifications, including patient preferences[82] as a fully holistic approach. To better understand and integrate patient preferences, novel endpoints need to be established that represent such preferences. Most likely, such a process needs to be guided by older patient representatives who support the process to develop provider- versus patient-relevant endpoints. The four major columns of such an approach and their interplay are depicted in Figure 1. Trials in geriatric oncology should aim at integration of all these aspects.
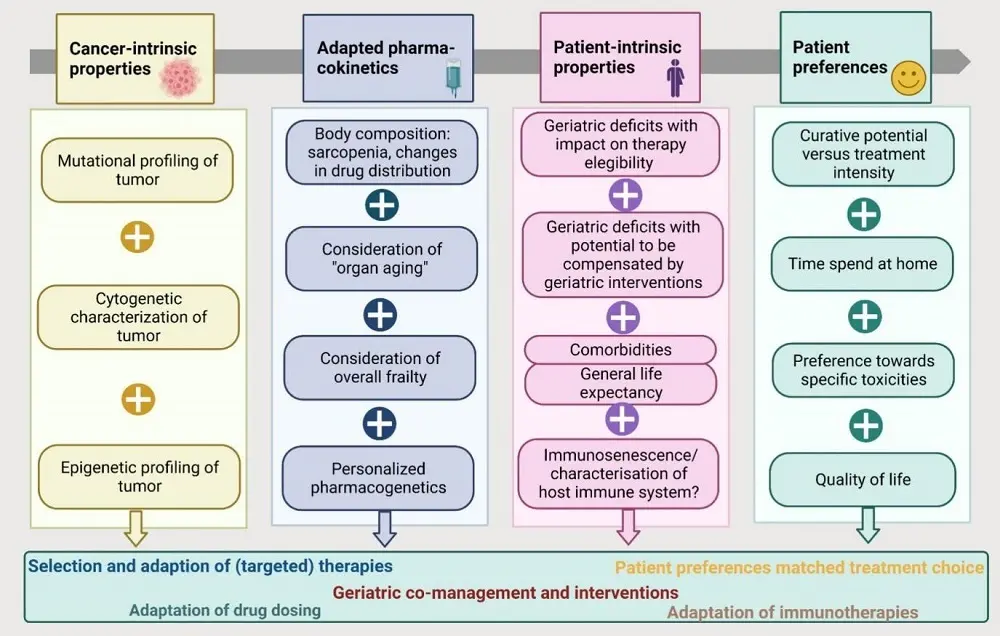
Figure 1. Integration of multiple layers of personalized cancer treatment at older age in future. These four major columns each represent an important field that should be integrated in future design of trials for older adults with cancer. As these fields require a broad range of diverse skills and specialities, multidisciplinary clinical and translational teams are needed. The figure was created with Biorender.
Authors contribution
Neuendorff NR, Christofyllakis K: Writing-original draft, conceptualization, data curation, formal analysis, manuscript editing.
Reinhardt HC: Manuscript editing, review for important intellectual content.
Conflicts of interest
Neuendorff NR has received honoraria and travel support by Janssen-Cilag, Medac, Novartis, Pfizer, Abbvie, and Jazz Pharmaceutical.
Christofyllakis K das received honoraria and travel support from Hexal, Celgene, Jazz Pharmaceuticals, AbbVie, Novartis and Roche, as well as research support from AstraZeneca.
Reinhardt HC reports research grants from the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe, The State of Northrhine-Westfalia, the Bundesministerium für Bildung und Forschung, the Else Kröner-Fresenius Stiftung and the MERCUR Stiftung. Additional research grants were obtained from AstraZeneca, Gilead, and SinABiomedics; consulting fees from BMS/Celgene, Novartis, Roche, Gilead; honoraria from AstraZeneca, Novartis, Roche AbbVie, Kite-Gilead, Merck Sharp & Dohme, Takeda, and Novartis. Reinhardt HC is a co-founder of CDL Therapeutics GmbH.
Neuendorff NR is an Editorial Board member of Ageing and Cancer Research & Treatment.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (DFG) in the framework of the DFG University Medicine Essen Clinician Scientist Academy (UMEA), FU 356/12-2 to NRN.
Copyright
© The Author(s) 2023.
References
-
1. Goede V, Neuendorff NR, Schulz RJ, Hormigo AI, Martinez-Peromingo FJ, Cordoba R. Frailty assessment in the care of older people with haematological malignancies. Lancet Healthy Longev. 2021;2(11):e736-e745.
[DOI] -
2. Van Herck, Feyaerts A, Alibhai S, Papamichael D, Decoster L, Lambrechts Y, et al. Is cancer biology different in older patients? Lancet Healthy Longev. 2021;2(10):e663-e677.
[DOI] -
3. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316.
[DOI] -
4. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904.
[DOI] -
5. Li D, Sun CL, Kim H, Soto-Perez-de-Celis E, Chung V, Koczywas M, et al. Geriatric assessment–driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158.
[DOI] -
6. Soo WK, King MT, Pope A, Parente P, Darzins P, Davis ID. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: a multicentre, open-label, randomised controlled trial. Lancet Healthy Longev. 2022;3(9):e617-e627.
[DOI] -
7. Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347.
[DOI] -
8. Dotan E, Walter LC, Browner IS, Clifton K, Cohen HJ, Extermann M, et al. NCCN guidelines® insights: older adult oncology, version 1.2021: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2021;19(9):1006-1019.
[DOI] -
9. Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300.
[DOI] -
10. Uranga C, Chien LC, Liposits G. Geriatric screening in older adults with cancer-a young international society of geriatric oncology and nursing & allied health interest group initiative. J Geriatr Oncol. 2022;13(3):374-377.
[DOI] -
11. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166-2172.
[DOI] -
12. Kenis C, Decoster L, Van Puyvelde K, De Grève J, Conings G, Milisen K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014;32(1):19-26.
[DOI] -
13. Oiwa K, Fujita K, Lee S, Morishita T, Tsukasaki H, Negoro E, et al. Utility of the geriatric 8 for the prediction of therapy-related toxicity in older adults with diffuse large B-cell lymphoma. Oncologist. 2021;26(3):215-223.
[DOI] -
13. Oiwa K, Fujita K, Lee S, Morishita T, Tsukasaki H, Negoro E, et al. Utility of the geriatric 8 for the prediction of therapy-related toxicity in older adults with diffuse large B-cell lymphoma. Oncologist. 2021;26(3):215-223.
[DOI] -
14. Lund CM, Vistisen KK, Olsen AP, Bardal P, Schultz M, Dolin TG, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Br J Cancer. 2021;124(12):1949-1958.
[DOI] -
15. Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691-1699.
[DOI] -
16. Pottel L, Boterberg T, Pottel H, Goethals L, Van Den Noortgate N, Duprez F, et al. Determination of an adequate screening tool for identification of vulnerable elderly head and neck cancer patients treated with radio (chemo) therapy. J Geriatr Oncol. 2012;3(1):24-32.
[DOI] -
17. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457.
[DOI] -
18. Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366.
[DOI] -
19. Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer. 2012;118(13):3377-3386.
[DOI] -
20. Battisti NML, Arora SP. An overview of chemotherapy toxicity prediction tools in older adults with cancer: A young international society of geriatric oncology and nursing and allied health initiative. J Geriatr Oncol. 2022;13(4):521-525.
[DOI] -
21. Kuon J, Hommertgen A, Krisam J, Lasitschka F, Stenzinger A, Blasi M, et al. Durvalumab in frail and elderly patients with stage four non-small cell lung cancer: study protocol of the randomized phase II DURATION trial. Trials. 2020;21:352.
[DOI] -
22. Ritchie EK, Klepin HD, Storrick E, Major B, Le-Rademacher J, Wadleigh M, et al. Geriatric assessment for older adults receiving less-intensive therapy for acute myeloid leukemia: report of CALGB 361101. Blood Adv. 2022;6(12):3812-3820.
[DOI] -
23. Klepin HD, Ritchie E, Major-Elechi B, Le-Rademacher J, Seisler D, Storrick L, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113.
[DOI] -
24. DuMontier C, Uno H, Hshieh T, Zhou G, Chen R, Magnavita ES, et al. Randomized controlled trial of geriatric consultation versus standard care in older adults with hematologic malignancies. Haematologica. 2022;107(5):1172-1180.
[DOI] -
25. Puts M, Alqurini N, Strohschein F, Koneru R, Szumacher E, Mariano C, et al. Impact of geriatric assessment and management on quality of life, unplanned hospitalizations, toxicity, and survival for older adults with cancer: the randomized 5C trial. J Clin Oncol. 2023;41(4):847-858.
[DOI] -
26. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601.
[DOI] -
27. Anjanappa M, Corden M, Green A, Roberts D, Hoskin P, McWilliam A, et al. Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50-57.
[DOI] -
28. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28-36.
[DOI] -
29. Maurus J, Terzer T, Benner A, Goisser S, Eidam A, Roth A, et al. Validation of a proxy-reported SARC-F questionnaire for current and retrospective screening of sarcopenia-related functional impairments. J Cachexia Sarcopenia Muscle. 2022;13(1):264-275.
[DOI] -
30. Zhao H, Cheng R, Song G, Teng J, Shen S, Fu X, et al. The effect of resistance training on the rehabilitation of elderly patients with sarcopenia: a meta-analysis. Int J Environ Res Public Health. 2022;19(23):15491.
[DOI] -
31. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. an SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10(5):956-961.
[DOI] -
32. Tan BHL, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333-338.
[DOI] -
33. Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015;23(3):687-694.
[DOI] -
34. Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920-2926.
[DOI] -
35. Matsunaga T, Saito H, Miyauchi W, Shishido Y, Miyatani K, Morimoto M, et al. Impact of skeletal muscle mass in patients with unresectable gastric cancer who received palliative first-line chemotherapy based on 5-fluorouracil. BMC Cancer. 2021;21(1):1219.
[DOI] -
36. Halliday LJ, Boshier PR, Doganay E, Wynter-Blyth V, Buckley JP, Moorthy K. The effects of prehabilitation on body composition in patients undergoing multimodal therapy for esophageal cancer. Dis Esophagus. 2023;36(2):doac046.
[DOI] -
37. Roche M, Ravot C, Malapert A, Paget-Bailly S, Garandeau C, Pitiot V, et al. Feasibility of a prehabilitation programme dedicated to older patients with cancer before complex medical–surgical procedures: the PROADAPT pilot study protocol. BMJ Open. 2021;11(4):e042960.
[DOI] -
38. Dolin TG, Mikkelsen M, Jakobsen HL, Nordentoft T, Pedersen TS, Vinther A, et al. Geriatric assessment and intervention in older vulnerable patients undergoing surgery for colorectal cancer: a protocol for a randomised controlled trial (GEPOC trial). BMC Geriatr. 2021;21(1):88.
[DOI] -
39. Collins KH, Herzog W, MacDonald GZ, Reimer RA, Rios JL, Smith IC, et al. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol. 2018;9:112.
[DOI] -
40. Crochow LB, Baraldi C, Noe D. Is dose normalization to weight or body surface area useful in adults? JNCI: J Natl Cancer Inst. 1990;82(4):323-325.
[DOI] -
41. Baker SD, Verweij J, Rowinsky EK, Donehower RC, Schellens JHM, Grochow LB, et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001. J Natl Cancer Inst. 2002;94(24):1883-1888.
[DOI] -
42. Williams GR, Outlaw D, Harvey RD, Lichtman SM, Zamboni WC, Giri S. Chemotherapy dosing in older adults with cancer: one size does not fit all. J Geriatr Oncol. 2023;14(1):101363.
[DOI] -
43. Hertz DL, Chen L, Henry NL, Griggs JJ, Hayes DF, Derstine BA, et al. Muscle mass affects paclitaxel systemic exposure and may inform personalized paclitaxel dosing. Br J Clin Pharmacol. 2022;88(7):3222-3229.
[DOI] -
44. Chargi N, Molenaar-Kuijsten L, Huiskamp LFJ, Devriese LA, de Bree R, Huitema ADR. The association of cisplatin pharmacokinetics and skeletal muscle mass in patients with head and neck cancer: the prospective PLATISMA study. Eur J Cancer. 2022;160:92-99.
[DOI] -
45. Drami I, Pring ET, Gould L, Malietzis G, Naghibi M, Athanasiou T, et al. Body composition and dose-limiting toxicity in colorectal cancer chemotherapy treatment; a systematic review of the literature. Could muscle mass be the new body surface area in chemotherapy dosing? Clin Oncol. 2021;33(12):e540-e552.
[DOI] -
46. Hall PS, Swinson D, Cairns DA, Waters JS, Petty R, Allmark C, et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(8):1249.
[DOI] -
47. Lund CM, Vistisen KK, Dehlendorff C, Rønholt F, Johansen JS, Nielsen DL. The effect of geriatric intervention in frail elderly patients receiving chemotherapy for colorectal cancer: a randomized trial (GERICO). BMC Cancer. 2017;17(1):448.
[DOI] -
48. Grant SJ, Mian HS, Giri S, Boutin M, Dottorini L, Neuendorff NR, et al. Transplant-ineligible newly diagnosed multiple myeloma: current and future approaches to clinical care: a young international society of geriatric oncology review paper. J Geriatr Oncol. 2021;12(4):499-507.
[DOI] -
49. Klepin HD, Estey E, Kadia T. More versus less therapy for older adults with acute myeloid leukemia: new perspectives on an old debate. Am Soc Clin Oncol Educ Book. 2019;39:421-432.
[DOI] -
50. Klepin HD, Neuendorff NR, Larson RA, Hamaker ME, Breccia M, Montesinos P, et al. Treatment of acute promyelocytic leukemia in older patients: recommendations of an International Society of Geriatric Oncology (SIOG) task force. J Geriatr Oncol. 2020;11(8):1199-1209.
[DOI] -
51. Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124.
[DOI] -
52. Neuendorff NR, Gagelmann N, Singhal S, Meckstroth S, Thibaud V, Zhao Y, et al. Hypomethylating agent-based therapies in older adults with acute myeloid leukemia–a joint review by the Young International Society of Geriatric Oncology and European Society for Blood and Marrow Transplantation Trainee Committee. J Geriatr Oncol. 2023;14(3):101406.
[DOI] -
53. Récher C, Röllig C, Bérard E, Bertoli S, Dumas PY, Tavitian S, et al. Long-term survival after intensive chemotherapy or hypomethylating agents in AML patients aged 70 years and older: a large patient data set study from European registries. Leukemia. 2022;36(4):913-922.
[DOI] -
54. Richardson DR, Zhou X, Jensen CE, Reeder-Hayes KE, Lund JE, Baggett C. Time at home among older adults with acute myeloid leukemia based on treatment intensity: A SEER-Medicare analysis. J Clin Oncol. 2022;40(16):6586.
[DOI] -
55. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
[DOI] -
56. Pratz KW, Panayiotidis P, Recher C, Wei X, Jonas BA, Montesinos P, et al. Venetoclax combinations delay the time to deterioration of HRQoL in unfit patients with acute myeloid leukemia. Blood Cancer J. 2022;12(4):71.
[DOI] -
57. Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531.
[DOI] -
58. Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104-2115.
[DOI] -
59. Facon T, Cook G, Usmani SZ, Hulin C, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077.
[DOI] -
60. Perrot A, Facon T, Plesner T, Usmani SZ, Kumar S, Bahlis NJ, et al. Health-related quality of life in transplant-ineligible patients with newly diagnosed multiple myeloma: findings from the phase III MAIA trial. J Clin Oncol. 2021;39(3):227-237.
[DOI] -
61. Li P, Yang X, Feng Y, Wu L, Ma W, Ding G, et al. The impact of immunosenescence on the efficacy of immune checkpoint inhibitors in melanoma patients: a meta-analysis. OncoTargets Ther. 2018;11:7521-7527.
[DOI] -
62. Kanesvaran R, Cordoba R, Maggiore R. Immunotherapy in older adults with advanced cancers: implications for clinical decision-making and future research. Am Soc Clin OncolEduc Book. 2018;38:400-414.
[DOI] -
63. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Gutiérrez EC, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24-34.
[DOI] -
64. Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276.
[DOI] -
65. Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-drug conjugates: the last decade. Pharmaceuticals. 2020;13(9):245.
[DOI] -
66. Fitzgerald L, Kittai A, Nastoupil LJ, Waller A, Jacobson CA, Saucieret A, et al. Real-world outcomes of elderly patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) treated with chimeric antigen receptor T-cell (CAR-T) therapy. J Clin Oncol. 2020;38:8039.
[DOI] -
67. Lin RJ, Lobaugh SM, Pennisi M, Chan HT, Batlevi Y, Ruiz JD, et al. Impact and safety of chimeric antigen receptor T-cell therapy in older, vulnerable patients with relapsed/refractory large B-cell lymphoma. Haematologica. 2021;106(1):255-258.
[DOI] -
68. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10307):314-324.
[DOI] -
69. Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716.
[DOI] -
70. Berdeja JG, Raje NS, Siegel DS, Lin Y, Anderson LD, Rodriguez-Otero P, et al. Efficacy and safety of idecabtagene vicleucel (ide-cel, bb2121) in elderly patients with relapsed and refractory multiple myeloma: karMMa subgroup analysis. Blood. 2020;136:16-17.
[DOI] -
71. Shouse G, Danilov AV, Artz A. CAR T-cell therapy in the older person: indications and risks. Curr Oncol Rep. 2022;24(9):1189-1199.
[DOI] -
72. Giri S, Bal S, Godby KN, Ravi G, Clark D, Ubersax C, et al. Real-world applicability of commercial chimeric antigen receptor T-cell therapy among older adults with relapsed and/or refractory multiple myeloma. Am J Hematol. 2022;97(4):E153-E155.
[DOI] -
73. Kaiser M, Semeraro MD, Herrmann M, Absenger G, Gerger A, Renner W. Immune aging and immunotherapy in cancer. Int J Mol Sci. 2021;22(13):7016.
[DOI] -
74. Wang C, Wang H, Wang L. Biomarkers for predicting the efficacy of immune checkpoint inhibitors. J Cancer. 2022;13(2):481-495.
[DOI] -
75. Moreira A, Gross S, Kirchberger MC, Erdmann M, Schuler G, Heinzerling L. Senescence markers: Predictive for response to checkpoint inhibitors. Int J Cancer. 2019;144(5):1147-1150.
[DOI] -
76. Ferrara R, Naigeon M, Auclin E, Duchemann B, Cassard L, Jouniaux JM, et al. Circulating T-cell immunosenescence in patients with advanced non–small cell lung cancer treated with single-agent PD-1/PD-L1 inhibitors or platinum-based chemotherapy immunosenescence in NSCLC. Clin Cancer Res. 2021;27(2):492-503.
[DOI] -
77. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Maciejewski JP, Nimer SD, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9-16.
[DOI] -
78. Neuendorff NR, Frenzel LP, Leuschner F, Rüthlein J, Bartels S, Kölbl H, et al. Integrating clonal haematopoiesis into geriatric oncology: the ARCH between aging, cardiovascular disease and malignancy. J Geriatr Oncol. 2021;12(3):479-482.
[DOI] -
79. BrintzenhofeSzoc K, Krok-Schoen JL, Canin B, Bak K, D'Andrea G, Hines C, et al. The underreporting of phase III chemotherapeutic clinical trial data of older patients with cancer: A systematic review. J Geriatr Oncol. 2020;11(3):369-379.
[DOI] -
80. Hopkins JO, Braun-Inglis C, Guidice S, Crews J, Davidson B, Thomas H, et al. Enrolling older adults onto National Cancer Institute-funded clinical trials in community oncology clinics: Barriers and solutions. J Natl Cancer Inst Monogr. 2022;2022(60):117-124.
[DOI] -
81. Le-Rademacher J, Mohile SG, Unger JM, Hall C, Lau D, Tan A, et al. Trial design considerations to increase older adult accrual to National Cancer Institute clinical trials. J Natl Cancer Inst Monogr. 2022;2022(60):135-141.
[DOI] -
82. Richardson DR, Loh KP. Improving personalized treatment decision-making for older adults with cancer: The necessity of eliciting patient preferences. J Geriatr Oncol. 2022;13(1):1-3.
[DOI]
Copyright
© The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher's Note
Share And Cite




