Abstract
Nitrogen/boron-based multi-resonance thermally activated delayed fluorescence (MR-TADF) materials offer advantages in terms of high photoluminescence quantum yield (PLQY) and narrowband emission, making them highly promising for display applications. Represented by ν-DABNA, diboron MR-TADF materials demonstrate the potential for high-efficiency narrowband emission. However, their large planar structures are susceptible to intermolecular interactions, thus increasing the complexity of device fabrication. In this research, our objective was to enhance the anti-aggregation capabilities of the diboron-based ν-DABNA by incorporating sterically hindered terphenyl groups. We synthesized two luminescent materials, DTPF-ν-DABNA and DTP-ν-DABNA, with intermolecular distances exceeding 4 Å in single-crystal stacking, significantly inhibiting intermolecular interactions. Both materials achieved an external quantum efficiency (EQE) exceeding 30% and full width at half maximum (FWHM) of no more than 22 nm, demonstrating characteristics of high-efficiency narrowband emission. Our research provides a straightforward and practical method to obtain MR-TADF materials with high device efficiency and low sensitivity to doping concentration.
Keywords
1. Introduction
Currently, organic light-emitting diodes (OLEDs)[1] have been widely used in the display industry. Among the emissive materials, thermally activated delayed fluorescence (TADF)[2-9] materials offer a cost-effective alternative to expensive noble metal complexes. TADF materials can achieve 100% internal quantum efficiency (IQE) through the process of reverse intersystem crossing (RISC) with pure organic materials. To achieve delayed fluorescence, the material must possess a sufficiently fast RISC rate (k RISC). This requires the molecule to have a small energy gap (ΔEST) between S1 and T1. Donor-acceptor (D-A) type TADF materials can achieve a small ΔEST by constructing twisted donor and acceptor structures that separate the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). However, molecules designed using this strategy often exhibit significant charge transfer (CT) characteristics and multiple vibrational levels, leading to substantial structural relaxation and a large stokes shift, which hampers the achievement of narrowband emission. Based on this fact, Hatakeyama et al. employed nitrogen atoms with electron-donating capabilities and boron atoms with electron-withdrawing abilities to construct a rigid polycyclic aromatic framework[10]. By utilizing the multi-resonance (MR) effect, they successfully separated the HOMO and LUMO, achieving a reduced ΔEST. MR-TADF materials feature a rigid molecular structure[11-18], enabling significant suppression of vibrational coupling and yielding a minimal a stokes shift, thereby facilitating small full width at half maximum (FWHM).
The emergence of MR-TADF materials has greatly expanded the variety of TADF materials. From initially incorporating single boron (B) atoms, the molecular design has gradually progressed to include diboron and even multiple B atoms in MR-TADF systems[19-29]. Comparatively, the diboron system with a larger π-conjugated plane exhibits superior optoelectronic performance compared to single B-based MR-TADF. Notably, Hatakeyama et al. designed a diboron emitter ν-DABNA[30], which demonstrates an extremely narrow spectrum (λ EL = 469 nm, FWHM = 18 nm) and high external quantum efficiency (EQE) (34.4%), offering novel insights for the design of MR-TADF materials. The ultra-high device efficiency and extremely narrow FWHM of ν-DABNA have attracted wide researchers' attention to this diboron system with large planar structures. Hatakeyama et al. introduced oxygen atoms into ν-DABNA, leading to the design and synthesis of ν-DABNA-O[31]. The incorporation of oxygen atoms restricted the π-conjugation of HOMO. Compared to ν-DABNA, ν-DABNA-O with oxygen atoms exhibited a larger HOMO-LUMO gap, enabling a bluer and narrower emission in device (λ EL = 465 nm, FWHM = 23 nm). Kwon et al. designed the methyl- and fluoro-substituted 4F-m-ν-DABNA[32], where the introduction of methyl and fluoro atoms lowered the HOMO energy level, increased the bandgap, and further blue-shifted the molecular spectra (λ EL = 461 nm, FWHM = 18 nm). The addition of methyl and fluoro atoms also acted as steric hindrance, to some extent inhibiting the π-π interactions between planes. Even at a doping concentration of 3 wt%, the molecule achieved a high EQE of 33.7% (0.5 wt% doping concentration for ν-DABNA). Yoo et al. introduced methyl groups as steric hindrance agents around ν-DABNA, designing the sterically encapsulated o-Tol-ν-DABNA-Me[33]. The introduced steric hindrance groups reduced van der Waals interactions between molecules while preserving the extensive conjugated structure of ν-DABNA. At a doping concentration of 3 wt%, an EQE of 33.1% was achieved, maintaining an FWHM of 18 nm. These studies demonstrate the developmental potential of ν-DABNA while highlighting its high sensitivity to doping concentration, which increases the difficulty in device fabrication.
In this study, we utilized terphenyl groups[34,35]with larger steric hindrance[36-38] as bulky substituents to modify the core of ν-DABNA, aiming to increase the molecular torsion degree. Consequently, we designed and synthesized two molecules, N7,N7,N13,N13,5,15-hexaphenyl-9,11-bis(2,6-difluorophenyl)-5,9,11,15-tetrahydro5,9,11,15-tetraaza-19b,20b-diboradinaphtho[3,2,1-de:1',2',3'-jk]pentacene-7,13-terphenyl (DTPF-ν-DABNA) and N7,N7,N13,N13,5,9,11,15-octaphenyl-5,9,11,15-tetrahydro-5,9,11,15-tetraaza-19b,20b-diboradinaphtho[3,2,1-de:1',2',3'-jk]pentacene-7,13-terphenyl (DTP-ν-DABNA), which are modified by peripheral terphenyl groups. From the single-crystal structures, it is observed that the introduced terphenyl groups exhibit torsion angles exceeding 50° with respect to the luminescent core. Notably, in DTPF-ν-DABNA, the distance between the faces exceeds 8 Å, demonstrating that the introduction of the terphenyl groups effectively inhibits intermolecular π-π interactions. Even when the doping concentration of DTPF-ν-DABNA increased from 2 wt% to 10 wt%, narrowband emission with a consistent FWHM of 21 nm was maintained. Furthermore, at a doping concentration of 5 wt%, the EQE remained 34.2%. These performance characteristics demonstrate that the introduced terphenyl groups are beneficial for enhancing the molecular anti-aggregation capabilities and improving the performance of the molecules at high doping concentrations. This provides a good approach for modifying MR-TADF materials with large planarity, such as ν-DABNA.
2. Experimental
2.1 Synthesis of DTPF-ν-DABNA and DTP-ν-DABNA
All the synthesis routes and details of DTPF-ν-DABNA and DTP-ν-DABNA are available in supplementary materials.
2.2 Characterization
The raw reagents, catalysts, and chemicals involved in the initial reaction were all obtained from commercial companies and used without further purification. Solvents used in the reaction, such as toluene, o-dichlorobenzene, etc. were purified by the solvents purification system. 1H NMR and 13C NMR spectra were recorded on a Bruker 400 spectrometer or a Bruker 600 spectrometer at room temperature. Mass spectra and time-of-Flight MS-MALDI (MALDI-TOF) were performed on a Thermo ISQ mass spectrometer using a direct exposure probe and Bruker Autoflex II/Compass 1.0, respectively.
The ultraviolet-visible (UV-vis) absorption spectra were measured by a Shimadzu UV-2600 spectrophotometer. Fluorescent, as well as phosphorescent spectra, were measured by a Hitachi F-4600 spectrophotometer. Thermogravimetric analysis (TGA) was measured by a METTLER TOLEDO TGA1 under a high purity nitrogen atmosphere.
The temperature was increased to 800 °C with a heating rate of 10 °C/min. Density functional theory (DFT) calculations were performed utilizing PBE0-6-31G (d, p) atomic basis set. Electrochemical analysis was achieved on a CHI 600D electrochemical workstation, and the scan rate was 100 mV S-1 at room temperature. The three-electrode configuration system in n-Bu4NPF6 (0.1 M) dichloromethane solution. The redox potential of ferrocene/ferrocenium (Fc/Fc+) was measured at room temperature. The photoluminescence quantum yield (PLQY) was measured using Hamamatsu C9920-02G in nitrogen or air atmosphere. Transient spectra were obtained by using the Quantaurus-Tau fluorescence lifetime measurement system (C11367-03, Hamamatsu Photonics Co.) in a vacuum.
2.3 Single crystal information
The crystal was grown by slow evaporation from dichloromethane and ethanol. We used a Bruker D8-Venture diffractometer with a Turbo X-ray Source (Mo-Kα radiation, λ = 0.71073 Å) adopting the direct-drive rotating anode technique and a CMOS detector to collect the single-crystal data. The structures were solved by direct methods and refined by the full-matrix least-squares on F2 using the SHELXTL-2014 program. The X-ray crystallographic coordinates for the structure reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers DTPF-ν-DABNA and DTP-ν-DABNA (2303137 and 2322055). These data can be obtained free of charge at The Cambridge Crystallographic Data Centre.
2.4 Devices fabrication and measurements
OLEDs were fabricated on the ITO glass substrate layers (110 nm, 15 Ω/square) under a base pressure of 3 × 10-6 Torr. The active area of each device was 0.09 cm2. Deposition rates and thicknesses of all materials were monitored using oscillating quartz crystals. The doping layer was deposited by utilizing two different sensors to monitor the deposition rates of both host material and dopant material. The deposition rate of the host was controlled at 0.2 nm s-1, and the deposition rate of the dopant was adjusted according to the volume ratio doped in the host materials. The electroluminescence (EL) and current density-voltage(J-V) characteristics of the devices were measured by a constant current source (Keithley 2400 SourceMeter) combined with a photometer (Photo Research SpectraScan PR655).
3. Results and Discussion
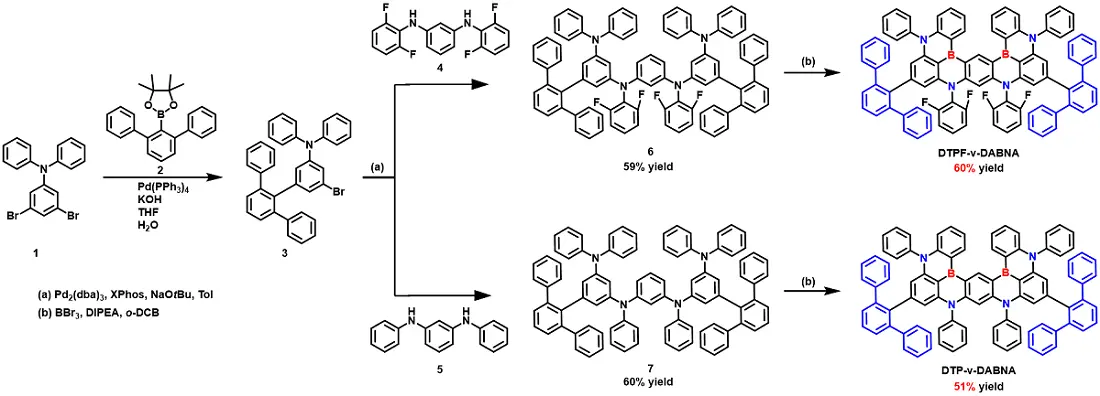
The synthesis routes for compounds DTPF-ν-DABNA and DTP-ν-DABNA are depicted in Figure 1, with detailed information provided in the supplementary materials. In the first step, a palladium-catalyzed Suzuki-Miyaura coupling reaction was designed to yield intermediate 3. The steric hindrance of the terphenyl groups prevents undesired one-shot borylation at unreactive positions, thus enhancing the overall yield of the target product. Subsequently, Buchwald-Hartwig coupling reactions were employed to generate precursors 6 and 7. Finally, the desired products, DTPF-ν-DABNA and DTP-ν-DABNA, were obtained via a one-shot borylation reaction at 200 °C. Both emitters demonstrate acceptable synthetic yields exceeding 50%, indicating that scaling up to commercial production would be easily achievable. All products were characterized by MALDI-TOF-MS and nuclear magnetic resonance (Figure S1,2,3,4,5). TGA reveals high decomposition temperatures for both molecules (Figure S6), measuring at 535 and 524 °C respectively, indicating excellent thermal and morphological stability.
To understand the influence of introducing terphenyl groups on the molecular structure and packing modes, single crystals of DTPF-ν-DABNA and DTP-ν-DABNA were obtained by slow evaporation of a mixture of ethanol and dichloromethane. The crystal structures were characterized using X-ray diffraction measurements (Table S1). Figure 2 reveals that the introduction of terphenyl groups increases the distortion in both DTPF-ν-DABNA and DTP-ν-DABNA. In DTP-ν-DABNA, the twisting angles between planes A2 and B2, as well as between planes C2 and D2, exceed 50°, indicating enhanced structural flexibility due to the introduction of terphenyl groups. In DTPF-ν-DABNA, the additional fluorine atoms along with the terphenyl groups further increase steric hindrance, resulting in twisting angles between planes A1 and B1, as well as between planes C1 and D1, reaching 59.83° and 59.03°, respectively. The distorted molecular structures caused by the introduction of terphenyl groups also lead to looser stacking in the single crystals. The presence of terphenyl groups increases the distance between planes, with maximum interplanar distances exceeding 8.00 Å in both DTPF-ν-DABNA and DTP-ν-DABNA (Figure S7). This impedes the contact between emitting planes and effectively suppresses aggregation-caused quenching. In the stacking of DTPF-ν-DABNA single crystals, the molecular arrangement is looser compared to DTP-ν-DABNA, with an intermolecular distance of 8.66 Å. This advantageous arrangement inhibits intermolecular interactions and enhances the molecule's resistance to aggregation. These findings demonstrate that the introduction of terphenyl groups induces a twisted molecular structure, which reduces the π-π stacking between the large planar structures in the solid state and minimizes energy losses due to face-to-face contacts.
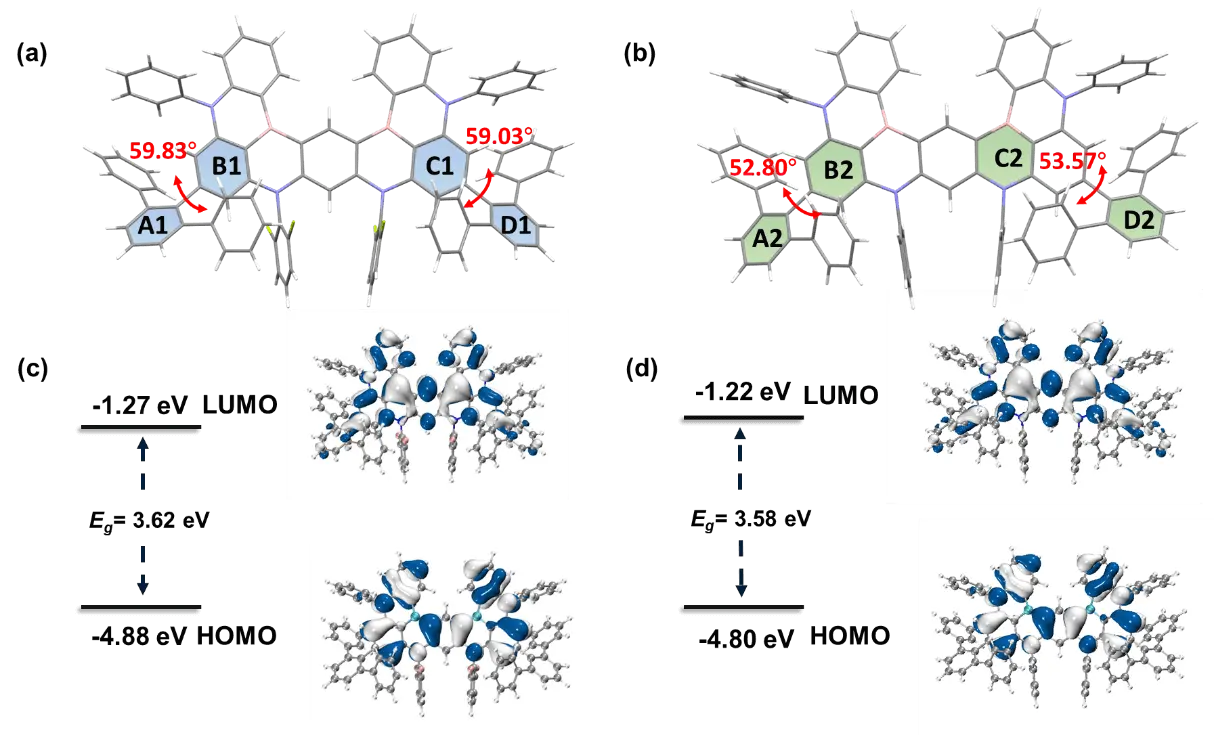
Figure 2. Crystal structures and calculation results of (a, c) DTPF-ν-DABNA and (b, d) DTP-ν-DABNA.
Extensive analyses of the molecular orbitals using DFT at the PBE0-6-31G (d, p) level were also conducted. The analysis revealed that the frontier molecular orbitals primarily localized on the MR core, as shown in Figure 2. The calculated values for the HOMO/LUMO/bandgap were -4.88/-1.27 eV for DTPF-ν-DABNA and -4.80/-1.22 eV for DTP-ν-DABNA. This indicates that the MR effect is not significantly influenced by the presence of the distorted terphenyl groups. However, it was observed that the LUMOs slightly extend onto the terphenyl groups in both compounds. Moreover, after replacing diphenylamine with terphenyl groups (Figure 3 and Figure S8 ), the bandgap becomes narrower, which may result in a redshift in the spectra of DTPF-ν-DABNA and DTP-ν-DABNA. Additionally, the introduction of electron-withdrawing fluorine atoms[32,39] slightly deepens the HOMO energy by 0.08 eV, thereby increasing the bandgap. As a result, it is possible that DTPF-ν-DABNA exhibits a blue shift compared to DTP-ν-DABNA in terms of its emission peak. The results of the excited states calculations indicate that the S1/T1 energies for DTPF-ν-DABNA and DTP-ν-DABNA are 2.93/2.51 eV and 3.00/2.60 eV, respectively. The holes and electrons are both distributed on the corresponding atoms, and the distribution of holes and electrons in S1 and T1 is similar (Figure S8 ). Furthermore, the excited-state calculations also reveal that the ΔEST for DTPF-ν-DABNA and DTP-ν-DABNA was larger than that of ν-DABNA (Figure S9).
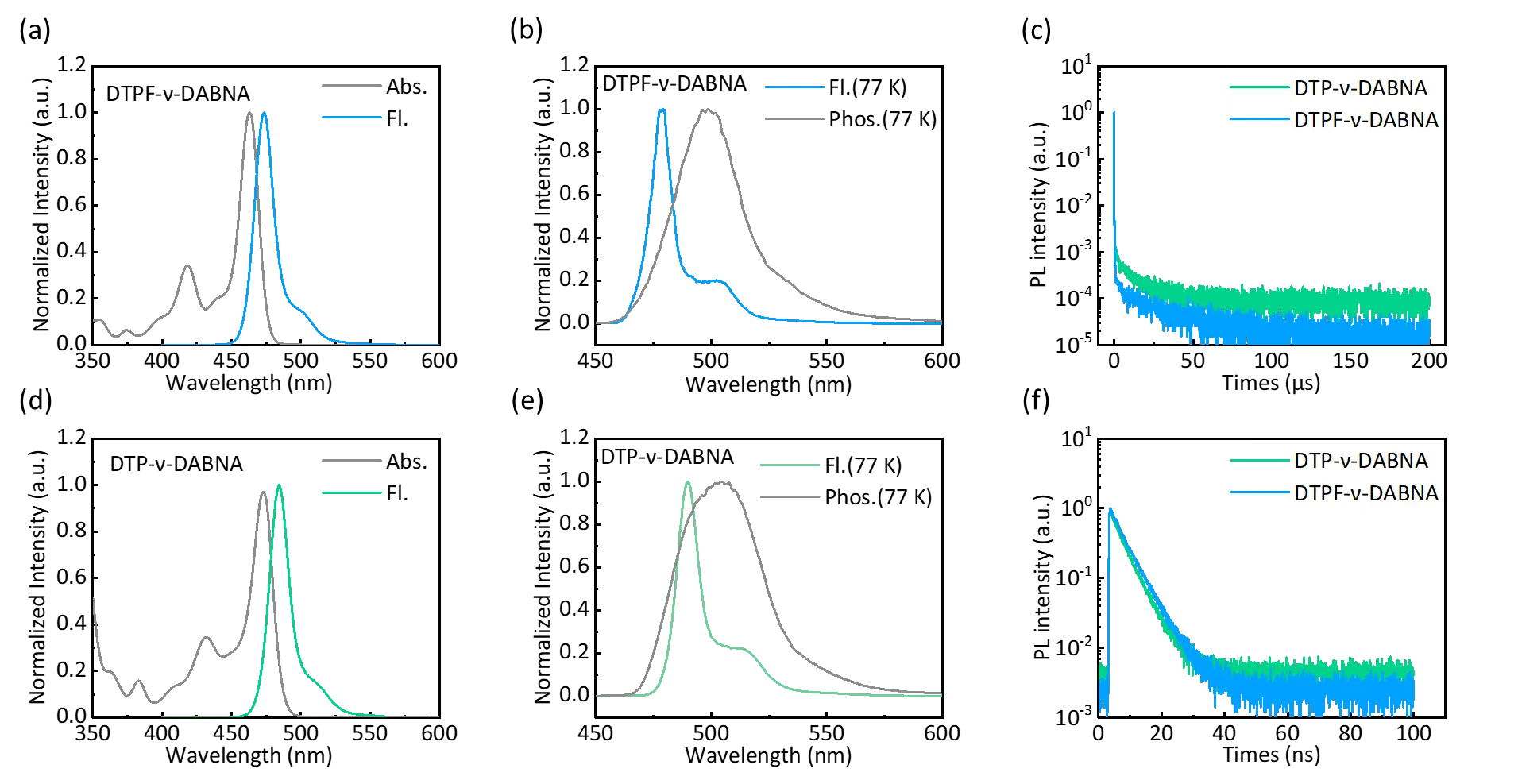
Figure 3. Absorption and fluorescence spectra of (a) DTPF-ν-DABNA and (d) DTP-ν-DABNA in dilute toluene solution (10-5 mol L -1) at room temperature. Fluorescence and phosphorescence spectra in dilute toluene solution (10-5 mol L -1) at 77 K of (b) DTPF-ν-DABNA and (e) DTP-ν-DABNA. Transient spectra of 2 wt% DTPF-ν-DABNA (c) and DTP-ν-DABNA (f) in PPF charge transfer (ICT) effect.
Subsequently, we estimated the energy levels of DTPF-ν-DABNA and DTP-ν-DABNA using differential pulse voltammetry (DPV) and cyclic voltammetry (CV) under a nitrogen atmosphere (Figure S10). The oxidation potential and reduction potential were determined from the first oxidation peak and first reduction peak in the DPV curves, respectively. The calculated HOMO and LUMO levels for DTPF-ν-DABNAwere -5.03 eV and -2.23 eV, while those for DTP-ν-DABNA were -4.98 eV and -2.36 eV, respectively. These results are consistent with the trends observed in the DFT calculations.
The photophysical properties of DTPF-ν-DABNA and DTP-ν-DABNA in solution are shown in Figure 3. In toluene at room temperature, both emitters exhibit symmetric absorption and emission spectra with minimal stokes shift and extremely narrowband emission (FWHM = 16/17 nm). This suggests the presence of non-bonding HOMO and LUMO in the molecules and the suppression of vibrational coupling in the S 1-S0 transition. Additionally, the introduction of terphenyl groups enhances the intramolecular interactions.
Compared to ν-DABNA (λ PL = 468 nm), both DTPF-ν-DABNA and DTP-ν-DABNA exhibit significant redshifts in their emission peaks, measured at 484 nm and 474 nm, respectively, which are consistent with theoretical calculations. Notably, DTP-ν-DABNA shows a larger redshift of 16 nm, while DTPF-ν-DABNA only experiences a redshift of 6 nm due to the presence of four electron-withdrawing fluorine atoms, which deepen the HOMO energy and enhance the bandgap (Table 1). Furthermore, pronounced solvatochromic effects were observed in both molecules (Figure S11 and Table S2), indicating the presence of significant charge transfer properties. To estimate the S1 and T1 states, low-temperature fluorescence and phosphorescence spectra of DTPF-ν-DABNA and DTP-ν-DABNA in toluene were further measured at 77 K. The calculated S1/T1 energies for DTPF-ν-DABNA were 2.59/2.49 eV, and for DTP-ν-DABNA, they were 2.53/2.43 eV. From these values, it can be determined that both molecules have ΔEST values of 0.1 eV, indicating the presence of TADF characteristics.
| Compound | λAbs(a) nm | λPL(a) nm | FWHM(a) nm | S1/T1(b) (eV) | ΔEST(c) (eV) | HOMO/LUMO(d) (eV) | Eg(e) (eV) | PLQY(f) (%) | Td(g) (°C) |
| ν-DABNA[21,30] | 457 | 468 | 14 | 2.73/2.71 | 0.02 | -5.28/-2.64 | 2.64 | 83.7 | 440 |
| 4F-ν-DABNA[32] | 447 | 457 | 14 | 2.84/2.79 | 0.05 | -5.66/-3.03 | 2.63 | 90.2 | 520 |
| DTPF-ν-DABNA | 463 | 474 | 16 | 2.59/2.49 | 0.1 | -5.03/-2.23 | 2.8 | 99.2 | 535 |
| DTP-ν-DABNA | 473 | 484 | 17 | 2.53/2.43 | 0.1 | -4.98/-2.36 | 2.62 | 95.5 | 524 |
(a) Photoluminescence maximum emission and full width at half maximum measured in toluene solution; (b) singlet and triplet energies estimated from the first peak of fluorescent and phosphorescent spectra in toluene matrix at 77 K; (c) ΔEST= S1-T1, (d) HOMO and LUMO obtained from cyclic voltammetry; (e) Eg= LUMO-HOMO; (f) PLQY in 2 wt% of doped film (1 wt% ν-DABNA in DOBNA-OAr and 3 wt% 4F-ν-DABNA in DBFPO); (g) decomposition temperature corresponding to 5% weight loss from TGA measurement. PLQY: photoluminescence quantum yield.
We further investigated the photophysical properties of DTPF-ν-DABNA and DTP-ν-DABNA in thin films. Even when doped at a concentration of 2% in a 3,3'-di(9H-carbazol-9-yl)-1,1'-biphenyl (PPF) film, DTPF-ν-DABNA, and DTP-ν-DABNA exhibited relatively narrow fluorescence spectra at 482 nm and 491 nm, with FWHM values of 19 nm and 23 nm, respectively (Figure S12 ). However, compared to their performance in toluene solution, a slight broadening and redshift were observed. Furthermore, both doped films of DTPF-ν-DABNA and DTP-ν-DABNA achieved high PLQY values of 99.2% and 95.5%, respectively. They also displayed short decay lifetimes and fast k RISC in transient photoluminescence decay measurements, reflecting significant TADF characteristics. The main kinetic parameters of DTPF-ν-DABNA and DTP-ν-DABNA are shown in Table S3. We also conducted transient spectra testing for DTPF-ν-DABNA and DTP-ν-DABNA at different temperatures (Figure S12 ). It was observed that the delayed component gradually increased as the temperature rose from 77 K to 300 K, demonstrating the TADF characteristics.
To further explore the device performance of these two MR-TADF emitters, devices based on DTPF-ν-DABNA and DTP-ν-DABNA were fabricated. The device structure was as follows: ITO/HAT-CN (10 nm)/TAPC (40 nm)/TCTA (10 nm)/mCP (10 nm)/ x wt% emitter in PPF (20 nm)/TmPyPb (40 nm)/Liq (2 nm)/Al (80 nm). Indium tin oxide (ITO) and aluminum (Al) were used as the anode and cathode, respectively. 1,4,5,8,9,11-hexaazaterphenylene hexacarbonitrile (HAT-CN) served as the hole-injection layer, while 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC) and tris(4-carbazolyl-9-ylphenyl)amine (TCTA) functioned as hole-transporting layers. 1,3-di(9H-carbazol-9-yl)benzene (mCP) acted as the electron-blocking layer, and PPF was used as the host material for the emitters. 1,3,5-tri[(3-pyridyl)phen-3-yl]benzene (TmPyPB) served as the electron-transporting layer. 8-hydroxyquinolinato lithium (Liq) was employed as the electron-injection layer. The energy level diagram and molecular structures of the aforementioned materials can be found in Figure S13.
As shown inFigure 4, DTPF-ν-DABNA and DTP-ν-DABNA achieved narrow emission peaks at 493 and 480 nm, respectively, with a FWHM of 22 and 21 nm. The CIE coordinates were (0.089, 0.225) and (0.068, 0.393) for DTPF-ν-DABNA and DTP-ν-DABNA, respectively. Both emitters exhibited device efficiencies exceeding 30%, with the maximum EQE of DTPF-ν-DABNA reaching 36.0% (Figure 4 and Figure S14). Although the efficiency decreased with increasing doping concentration, this is a common issue for molecules with large planar structures (Table S4 and Table S5). However, even with higher doping concentrations, DTPF-ν-DABNA and DTP-ν-DABNA maintained narrow emission spectra, indicating that the introduction of terphenyl groups did not disrupt their inherent rigid structures. When the doping concentration was increased from 2 wt% to 5 wt%, DTPF-ν-DABNA still maintained an EQE of 34.2% (Table 2). This can be attributed to the increased steric hindrance provided by the terphenyl and fluorine atoms, which more effectively suppress aggregation. Under the condition of a 10 wt% doping concentration, both DTPF-ν-DABNA and DTP-ν-DABNA exhibited EQE exceeding 20%, demonstrating that the terphenyl groups, as steric hindrance moieties, can to some extent enhance the molecule's resistance to aggregation. However, compared to ν-DABNA, these two materials exhibit severe efficiency roll-off (Table S4 ), which is attributed to a lower k RISC (Table S3) than that of ν-DABNA (2.0 × 105 s -1). Additionally, we have also conducted lifetime testing for DTPF-ν-DABNA and DTP-ν-DABNA, as shown in Figure S15.
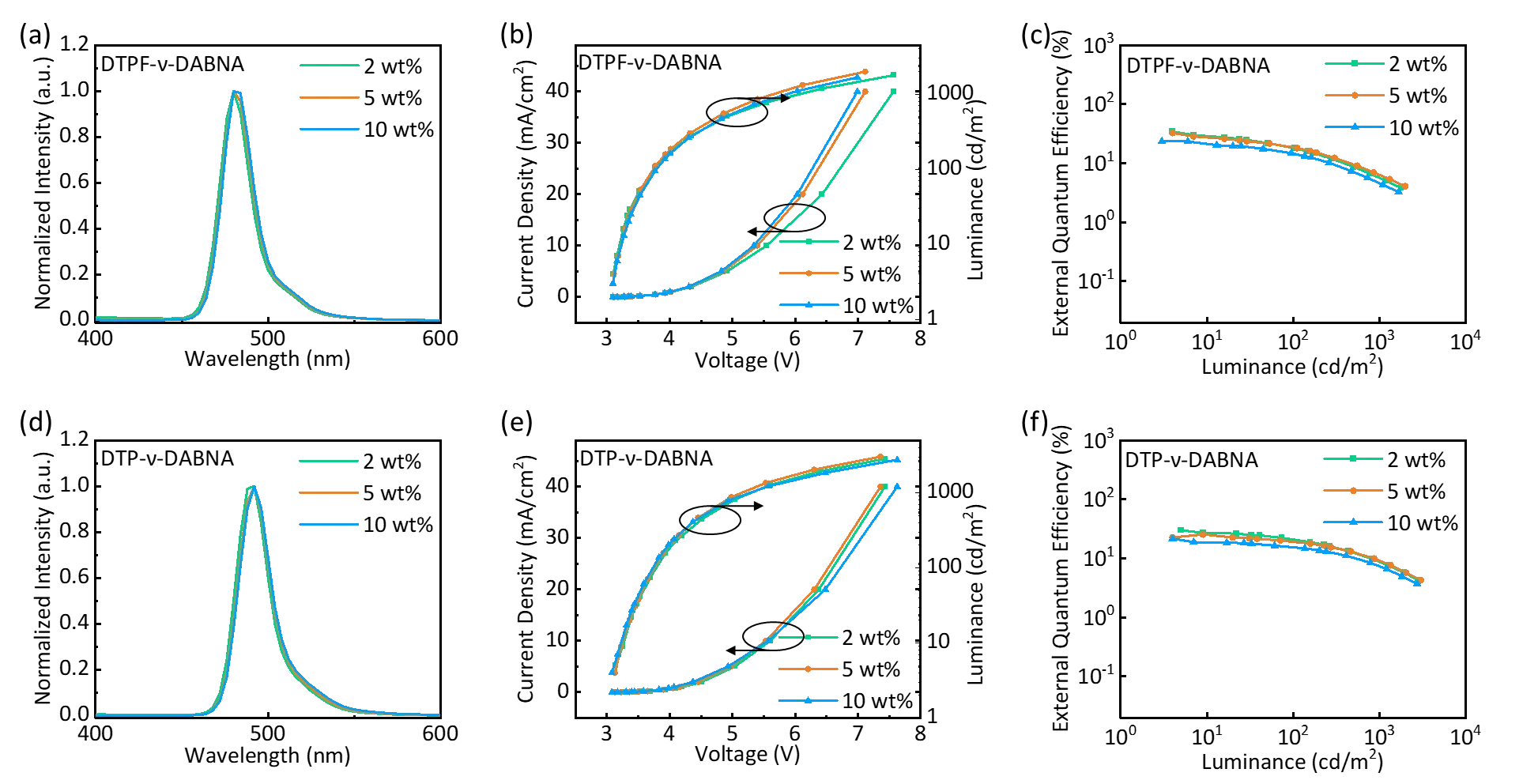
Figure 4. EL characteristics of devices based on DTPF-ν-DABNA and DTP-ν-DABNA: (a, d) EL spectra; (b, e) current density and luminance versus voltage characteristics; (c, f) EQE-luminance curves. EL: electroluminescence; EQE: external quantum efficiency.
| Dopant | x (wt%) | V (a) (V) | λ(b) (nm) | FWHM(c) (nm) | CE (d) (cd A-1) | PE(e) (lm W-1) | EQE(f) (%) | Luminance(g) (cd/m2) | CIE(h) (x, y) |
| DTPF-ν-DABNA | 2 | 3.1 | 480 | 21 | 40.0 | 40.5 | 36.0 | 1,783 | (0.092, 0.216) |
| 5 | 3.1 | 480 | 21 | 40.0 | 40.4 | 34.2 | 1,993 | (0.091, 0.223) | |
| 10 | 3.1 | 480 | 21 | 30.0 | 30.4 | 24.6 | 1,672 | (0.090, 0.238) | |
| DTP-ν-DABNA | 2 | 3.1 | 492 | 22 | 50.0 | 50.0 | 31.5 | 2,835 | (0.071, 0.380) |
| 5 | 3.1 | 492 | 22 | 44.9 | 43.7 | 25.6 | 3,023 | (0.070, 0.411) | |
| 10 | 3.1 | 492 | 22 | 40.0 | 40.8 | 22.7 | 2,749 | (0.076, 0.432) |
(a) Onset voltages at current density of 0.01 mA cm-2; (b) electroluminescence peak wavelength; (c) full-width at half-maximum; (d) maximum current efficiency; (e) maximum power efficiency; (f) maximum external quantum efficiency; (g) maximum luminance; (h) commission Internationale de l'Eclairage coordinates, recorded at 40 mA cm -2.
4. Conclusion
In summary, we designed a strategy to increase molecular distortion by incorporating terphenyl groups onto the diboron-based MR emitting core. This modification can increase the degree of molecular distortion, enabling efficient and narrowband emission in devices. The introduction of terphenyl groups imparts unique twisting structures to the molecules, with torsion angles exceeding 50° between the terphenyl and diboron core. Both DTPF-ν-DABNA and DTP-ν-DABNA exhibit intermolecular distances of over 4 Å in single stacks, indicating enhanced π-π interaction suppression conferred by the incorporation of terphenyl groups. In terms of device performance, DTPF-ν-DABNA and DTP-ν-DABNA both demonstrate superior characteristics. The EQEmax of DTPF-ν-DABNA and DTP-ν-DABNA exceeds 30%, maintaining an EQE above 20.0% even at a doping concentration of 10 wt%. Furthermore, there is no change in the FWHM or redshift in the electroluminescence spectra of DTPF-ν-DABNA and DTP-ν-DABNA when the doping concentration increases from 2 wt% to 10 wt%. This suggests that the terphenyl-encapsulated DTPF-ν-DABNA and DTP-ν-DABNA are less sensitive to doping concentration. Moreover, this strategy can be extended to other luminescent materials such as nitrogen carbonyl systems and indolo[3,2,1-jk]carbazole systems to suppress aggregation and achieve narrowband emission at different doping concentrations, thereby enhancing material performance.
Supplementary materials
The supplementary material for this article is available at: Supplementary materials.
Acknowledgements
This work is supported by the Suzhou Key Laboratory of Functional Nano & Soft Materials, the Collaborative Innovation Center of Suzhou Nano Science & Technology, the 111 Project, and the Joint International Research Laboratory of Carbon-Based Functional Materials and Devices. We are grateful for the assistance of Professor Chuo-Luo Yang from the College of Materials Science and Engineering of Shenzhen University in transient photoluminescence decay measurements.
Authors contribution
Liu FM: Wrote the paper, performed the experiments.
Jiang ZQ, Liao LS: Conceived and designed the experiments.
Qu ZH, Zuo P, Yang YJ, Wu JR, Zhou DY: Performed the experiments.
All authors have given approval to the final version of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval and consent
Not applicable.
Availability of data and materials
The data and materials could be obtained from the corresponding author upon request.
Funding
The authors acknowledge financial support from the National Natural Science Foundation of China (51873139, 22175124, 62175171, and 61961160731) and the Natural Science Foundation of Jiangsu Province of China (BK20220057).
Copyright
© The Author(s) 2024.
References
-
1. Tang CW, Vanslyke SA. Organic electroluminescent diodes. Appl Phys Lett. 1987;51(12):913-915.
[DOI] -
2. Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature. 2012;492(7428):234-238.
[DOI] -
3. Liu FM, Ding LY, Yu YJ, Li MT, Liao LS, Jiang ZQ. Toward narrowband emission: the chemical strategies of modifying boron-based luminescent materials. J Mater Chem C. 2023;11(34):11425-11439.
[DOI] -
4. Zuo P, Qu YK, Zheng Q, Liao LS, Jiang ZQ. Sensitized organic light-emitting diodes: towards high efficiency and long lifetimes. Mater Chem Front. 2023;7(9):1760-1780.
[DOI] -
5. Yu YJ, Liu FM, Meng XY, Ding LY, Liao LS, Jiang ZQ. Carbonyl-containing thermally activated delayed fluorescence emitters for narrow-band electroluminescence. Chemistry. 2023;29(5):e202202628.
[DOI] [PubMed] -
6. Yang SY, Qu YK, Liao LS, Jiang ZQ, Lee ST. Research progress of intramolecular pi-stacked small molecules for device applications. Adv Mater. 2022;34(22):e2104125.
[DOI] [PubMed] -
7. Qu YK, Zheng Q, Fan J, Liao LS, Jiang ZQ. Spiro compounds for organic light-emitting diodes. Acc Mater Res. 2021;2(12):1261-1271.
[DOI] -
8. Li HC, Tang X, Yang SY, Qu YK, Jiang ZQ, Liao LS. Spatial donor/acceptor architecture for intramolecular charge-transfer emitter. Chin Chem Lett. 2021;32(3):1245-1248.
[DOI] -
9. Khan A, Tang X, Zhong C, Wang Q, Yang SY, Kong FC, et al. Intramolecular-locked high efficiency ultrapure violet-blue (CIE-y < 0.046) thermally activated delayed fluorescence emitters exhibiting amplified spontaneous emission. Adv Funct Mater. 2021;31(15):2009488.
[DOI] -
10. Hatakeyama T, Shiren K, Nakajima K, Nomura S, Nakatsuka S, Kinoshita K, et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect. Adv Mater. 2016;28(14):2777-2781.
[DOI] [PubMed] -
11. Xu YC, Cheng Z, Li ZQ, Liang BY, Wang JX, Wang JB, et al. Molecular-structure and device-configuration optimizations toward highly efficient green electroluminescence with narrowband emission and high color purity. Adv Opt Mater. 2020;8(9):1902142.
[DOI] -
12. Zhang Y, Zhang D, Wei J, Hong XC, Lu Y, Hu DP, et al. Achieving pure green electroluminescence with CIEy of 0.69 and EQE of 28.2% from an aza-fused multi-resonance emitter. Angew Chem Int Ed Engl. 2020;59(40):17499-17503.
[DOI] [PubMed] -
13. Uemura S, Oda S, Hayakawa M, Kawasumi R, Ikeda N, Lee YT, et al. Sequential multiple borylation toward an ultrapure green thermally activated delayed fluorescence material. J Am Chem Soc. 2023;145(3):1505-1511.
[DOI] [PubMed] -
14. Yu YJ, Feng ZQ, Meng XY, Chen L, Liu FM, Yang SY, et al. Introducing spiro-locks into the nitrogen/carbonyl system towards efficient narrowband deep-blue multi-resonance TADF emitters. Angew Chem Int Ed Engl. 2023;62(40):e202310047.
[DOI] [PubMed] -
15. Luo XF, Song SQ, Ni HX, Ma H, Yang D, Ma D, et al. Multiple-resonance-induced thermally activated delayed fluorescence materials based on indolo3,2,1-jk]carbazole with an efficient narrowband pure-green electroluminescence. Angew Chem Int Ed Engl. 2022;61(41):e202209984.
[DOI] [PubMed] -
16. Yuan Y, Tang X, Du XY, Hu Y, Yu YJ, Jiang ZQ, et al. The design of fused amine/carbonyl system for efficient thermally activated delayed fluorescence: novel multiple resonance core and electron acceptor. Adv Opt Mater. 2019;7(7):1801536.
[DOI] -
17. Jin J, Wang S, Jiang H, Wang LX, Wong WY. Peripheral selenium modification of multi-resonance thermally activated delayed fluorescence molecules for high-performance blue organic light-emitting diodes. Adv Opt Mater. 2024;12(11):2302354.
[DOI] -
18. Jiang H, Jin J, Wong WY. High-performance multi-resonance thermally activated delayed fluorescence emitters for narrowband organic light-emitting diodes. Adv Funct Mater. 2023;33(50):2306880.
[DOI] -
19. Li B, Lou J, Zhang B, Liu L, He X, Xu H, et al. Modulating electronic confinement and structural distortion of multiple resonance emitters enables high-performance ultrapure blue OLED. Chem Eng J. 2024;482:148876.
[DOI] -
20. Hu JJ, Wei Y, Wang XZ, Liang X, Liao XJ, Yuan L, et al. Efficient ultra-narrowband OLEDs based on carbazole-fused dual-boron embedded multi-resonance thermally activated delayed fluorescence materials. Adv Opt Mater. 2024;12(15):2302987.
[DOI] -
21. Zhang K, Wang X, Chang Y, Wu Y, Wang S, Wang L. Carbazole-decorated organoboron emitters with low-lying HOMO levels for solution-processed narrowband blue hyperfluorescence OLED devices. Angew Chem Int Ed Engl. 2023;62(47):e202313084.
[DOI] [PubMed] -
22. Fan XC, Wang K, Shi YZ, Cheng YC, Lee YT, Yu J, et al. Ultrapure green organic light-emitting diodes based on highly distorted fused π-conjugated molecular design. Nat Photonics. 2023;17(3):280-285.
[DOI] -
23. Oda S, Sugitani T, Tanaka H, Tabata K, Kawasumi R, Hatakeyama T. Development of pure green thermally activated delayed fluorescence material by cyano substitution. Adv Mater. 2022;34(34):2201778.
[DOI] [PubMed] -
24. Oda S, Kawakami B, Yamasaki Y, Matsumoto R, Yoshioka M, Fukushima D, et al. One-shot synthesis of expanded heterohelicene exhibiting narrowband thermally activated delayed fluorescence. J Am Chem Soc. 2022;144(1):106-112.
[DOI] [PubMed] -
25. Fan XC, Huang F, Wu H, Wang H, Cheng YC, Yu J, et al. A quadruple-borylated multiple-resonance emitter with para/meta heteroatomic patterns for narrowband orange-red emission. Angew Chem Int Ed Engl. 2023;62(35):e202305580.
[DOI] [PubMed] -
26. Huang F, Fan XC, Cheng YC, Wu H, Xiong X, Yu J, et al. Combining carbazole building blocks and nu-DABNA heteroatom alignment for a double boron-embedded MR-TADF emitter with improved performance. Angew Chem Int Ed Engl. 2023;62(32):e202306413.
[DOI] [PubMed] -
27. Naveen KR, Oh JH, Lee HS, Kwon JH. Tailoring extremely narrow FWHM in hypsochromic and bathochromic shift of polycyclo-heteraborin MR-TADF materials for high-performance OLEDs. Angew Chem Int Ed Engl. 2023;62(32):e202306768.
[DOI] [PubMed] -
28. Lei B, Huang Z, Li S, Liu J, Bin Z, You J. Medium-ring strategy enables multiple resonance emitters with twisted geometry and fast spin-flip to suppress efficiency roll-off. Angew Chem Int Ed Engl. 2023;62(12):e202218405.
[DOI] [PubMed] -
29. Jin J, Duan C, Jiang H, Tao P, Xu H, Wong WY. Integrating asymmetric O-B-N unit in multi-resonance thermally activated delayed fluorescence emitters towards high-performance deep-blue organic light-emitting diodes. Angew Chem Int Ed Engl. 2023;62(18):e202218947.
[DOI] [PubMed] -
30. Kondo Y, Yoshiura K, Kitera S, Nishi H, Oda S, Gotoh H, et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat Photonics. 2019;13(10):678-682.
[DOI] -
31. Tanaka H, Oda S, Ricci G, Gotoh H, Tabata K, Kawasumi R, et al. Hypsochromic shift of multiple-resonance-induced thermally activated delayed fluorescence by oxygen atom incorporation. Angew Chem Int Ed Engl. 2021;60(33):17910-17914.
[DOI] [PubMed] -
32. Naveen KR, Lee H, Braveenth R, Yang KJ, Hwang SJ, Kwoin JH. Deep blue diboron embedded multi-resonance thermally activated delayed fluorescence emitters for narrowband organic light emitting diodes. Chem Eng J. 2022;432(7428):134381.
[DOI] -
33. Kim HS, Cheon HJ, Lee D, Lee W, Kim J, Kim YH, et al. Toward highly efficient deep-blue OLEDs: Tailoring the multiresonance-induced TADF molecules for suppressed excimer formation and near-unity horizontal dipole ratio. Sci Adv. 2023;9(22):eadf1388.
[DOI] [PubMed] [PMC] -
34. Ha J, Kim SC, Jung M, Lee JY. Rational design of blocking groups for high triplet energy n-type host materials. J Mater Chem C. 2022;10(15):5962-5969.
[DOI] -
35. Liu FM, Qu ZH, Zuo P, Yu YJ, Li MT, Liao LS, et al. Ternary wrapped nitrogen/carbonyl multiresonance TADF emitters with quenching-resistant abilities. ACS Mater Lett. 2024;6(4):1380-1387.
[DOI] -
36. Zhang Y, Wei J, Zhang D, Yin C, Li G, Liu Z, et al. Sterically wrapped multiple resonance fluorophors for suppression of concentration quenching and spectrum broadening. Angew Chem Int Ed Engl. 2022;61(2):e202113206.
[DOI] [PubMed] -
37. Qu YK, Zhou DY, Kong FC, Zheng Q, Tang X, Zhu YH, et al. Steric modulation of spiro structure for highly efficient multiple resonance emitters. Angew Chem Int Ed Engl. 2022;61(22):e202201886.
[DOI] [PubMed] -
38. Jiang P, Miao J, Cao X, Xia H, Pan K, Hua T, et al. Quenching-resistant multiresonance TADF emitter realizes 40% external quantum efficiency in narrowband electroluminescence at high doping level. Adv Mater. 2022;34(3):2106954.
[DOI] [PubMed] -
39. Oda S, Kawakami B, Horiuchi M, Yamasaki Y, Kawasumi R, Hatakeyama T. Ultra-narrowband blue multi-resonance thermally activated delayed fluorescence materials. Adv Sci. 2022;10(1):e2205070.
[DOI] [PubMed] [PMC]
Copyright
© The Author(s). This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher's Note
Share And Cite