The production of hydrogen (H2) via electrocatalytic water splitting is a promising approach for reducing reliance on fossil fuels and promoting sustainable development[1-3]. In this context, the development of anion exchange membrane water electrolyzers for practical overall water splitting has emerged as a key research focus in electrochemical energy conversion[4-6]. Given the scarcity of freshwater resources and the rapid expansion of renewable energy sources such as wind and solar power, seawater electrolysis driven by renewable electricity has attracted considerable attention (Figure 1a)[7-10]. In practical electrolysis, research has traditionally focused on the anode due to challenges such as corrosion and competing side reactions[11,12]. Simultaneously, the cathode is polarized to a reducing potential to drive the hydrogen evolution reaction (HER), and this applied potential inherently offers electrochemical protection, mitigating oxidative degradation and corrosion caused by reactive species in the seawater electrolyte[13,14]. A frequently overlooked factor, however, is that electricity from renewable sources is often intermittent, leading to repeated start-up and shutdown cycles in seawater electrolysis systems. During these off periods, the cathode is no longer maintained at a protective reducing potential, resulting in significant catalyst oxidation and posing a critical challenge to long-term stability under harsh operating conditions.
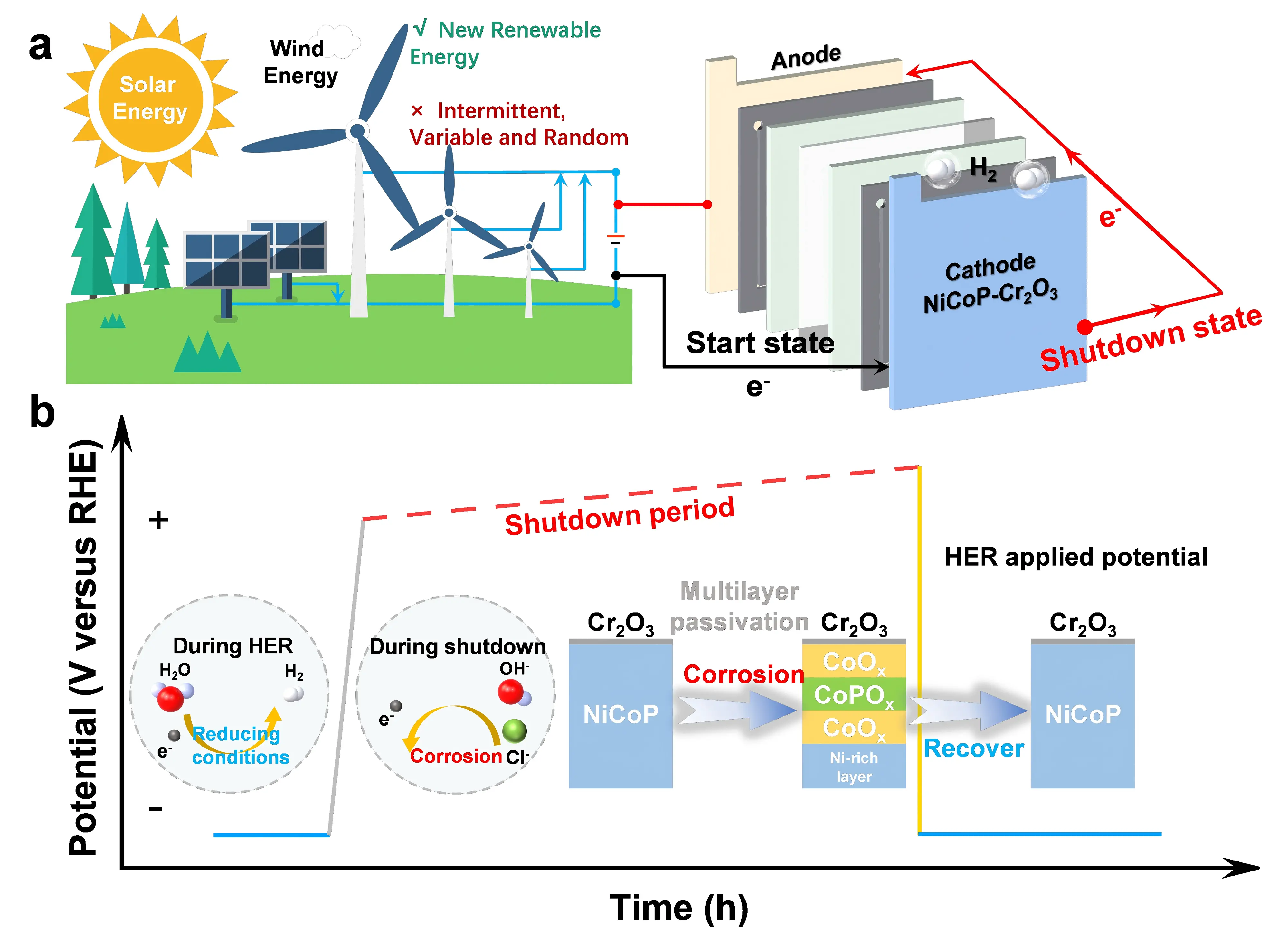
Figure 1. (a) Schematic of an alkaline seawater electrolyzer illustrating the start and shutdown states powered by renewable energy; (b) Schematic illustrating the HER process during operation and the passivation/recovery mechanism during shutdown/start at a corrosive potential in intermittent electrolysis. HER: hydrogen evolution reaction.
To address this challenge, Sha et al. in a seminal report published in Nature, elucidated the dynamic degradation process of cathodes during intermittent seawater electrolysis[15]. Simulations of repeated start-up and shutdown cycles revealed the presence of discharge and oxidation currents at the cathode, which led to the gradual degradation of catalytic activity. Furthermore, halide ions from seawater were found to readily adsorb onto the cathode surface under shutdown and discharge conditions, resulting in catalyst corrosion and poisoning. To quantitatively investigate the oxidation of the cathode, chronopotentiometric measurements were conducted. The cathode was polarized at an industrial-level current density, exhibiting a negative voltage; however, after electrolysis ceased, the voltage rapidly reversed, reaching 1.09 V after 12 hours and gradually increasing with longer shutdown durations (Figure 1b). These results indicate that, upon shutdown, a prolonged cathodic discharge process occurs, in which electrons are released and the cathode catalyst undergoes oxidation rather than the intended reduction reaction, ultimately causing irreversible oxidative damage. Concurrently, halide anions accumulated at the cathode, inducing corrosion of both the cathode and the current collector. The researchers successfully reproduced and analyzed this intermittent seawater electrolysis behavior using two electrochemical workstations, providing valuable insights for addressing practical electrolysis challenges.
Building on this insight, Sha et al. introduced a novel strategy involving the in-situ formation of catalyst passivation layers to maintain stable hydrogen evolution activity[15]. To mitigate severe cathode degradation during intermittent seawater electrolysis, a robust protection approach was developed, leveraging the synergistic effects of phosphorus and chromium(III) oxide (Cr2O3). Phosphorus, due to its strong affinity for oxygen, is readily oxidized during the discharge process and subsequently coordinates with up to four oxygen atoms to form an in-situ-generated phosphate passivation layer, which serves as the primary barrier against oxidation. The protective function of this phosphate layer is further enhanced by an outer Cr2O3 overlayer. Known for its exceptional stability under high-potential alkaline conditions, Cr2O3 not only densifies the overall passivation structure but also provides strong resistance against oxidation of the underlying metal sites by transmembrane oxygen, referring to oxygen ions that diffuse through the lattice via solid-state migration, similar to selective transport across biological membranes. The resulting combination of phosphate and Cr2O3 passivation layers was applied to the HER cathode, effectively shielding it from oxidative and corrosive damage during repeated start-up and shutdown cycles. Upon restart, the phosphate passivation layer dynamically recovers, creating a cycle that repeats with each subsequent shutdown. This redox-cyclic passivation mechanism represents an innovative strategy for addressing the challenges associated with intermittent operation in seawater electrolysis.
The practical viability of the NiCoP-Cr2O3 cathode for renewable-electricity-driven seawater electrolysis was evaluated through rigorous intermittent operation tests. In a full-cell configuration with a NiCoFeP anode, the system demonstrated outstanding durability, maintaining a current density of 0.5 A cm-2 for 10,000 hours in alkaline seawater under 12-hour start-up and shutdown cycles. An exceptionally low activity decay rate of only 0.5% khr-1 was recorded. This remarkable performance surpasses that of most previously reported seawater electrolysis systems. For example, the CoPi@CoP electrocatalyst reported by Liang et al., designed to buffer excess protons and prevent catalyst corrosion and active site blocking, remained stable for only 400 hours, whereas the NiCoP-Cr2O3 catalyst achieved an impressive 10,000 hours of operation[16]. This striking difference in durability motivated a detailed mechanistic study to uncover the fundamental basis for its exceptional resilience under intermittent operation.
Post-shutdown analysis of the sample revealed the formation of a passivation layer with a multilayered architecture. The outermost surface consisted of a mixed oxide phase of CoO and Cr2O3. Beneath this layer was an amorphous phosphate layer, under which a high-contrast cubic CoO lattice was observed. The innermost region retained the original NiCoP lattice structure. Electron energy loss spectroscopy (EELS) analysis further confirmed this structural transformation, showing the oxidation of cobalt species from the NiCoP phase to CoO. Concurrently, significant elemental redistribution occurred, resulting in the formation of a Ni-rich inner layer, while P species diffused to an intermediate sublayer, leading to the formation of a cobalt phosphate phase. This process ultimately produced a dense, three-layered oxide structure (Figure 1b). These oxide layers protect the underlying Ni, and the outermost Cr2O3 layer provides corrosion resistance under high-potential alkaline conditions. Such a multilayer oxide catalyst design has been rarely reported.
The reversibility of the stratified oxidation layer formed during shutdown is a critical factor for the long-term performance of the material. To investigate this, high-angle annular dark-field scanning transmission electron microscopy analysis was conducted on the NiCoP-Cr2O3 sample after multiple start-up and shutdown electrolysis cycles. The results showed that the hexagonal lattice of nickel-cobalt phosphide remained identical to the structure observed immediately before shutdown, demonstrating that the stratified passivation layer undergoes dynamic recovery under a reductive potential. Furthermore, comparison of the EELS spectra from the post-cycle and post-HER samples revealed no significant change in the cobalt valence state, indicating that the chemical state of cobalt within the NiCoP-Cr2O3 catalyst remains stable over numerous electrolysis cycles. The in-situ formation of oxide layers during shutdown prevents further catalyst oxidation, while these layers can be dynamically recovered under reducing potentials during electrolysis. This operating mechanism provides valuable insights for catalyst design. In addition, theoretical calculations revealed a sequential oxidation mechanism in NiCoP-Cr2O3, initiated by the attack and diffusion of OH- ions at the surface, which leads to the preferential oxidation of Co and P to form protective passivation layers. These layers not only create a high energy barrier that prevents deep oxidation but also resist chloride ion adsorption, collectively ensuring the successful reactivation of the catalyst in subsequent hydrogen evolution cycles.
In summary, the work by Sha et al. highlights the in-situ formation of multilayer passivation layers during shutdown periods as a key design principle for achieving long-term stability in intermittent alkaline seawater electrolysis[15]. This self-protecting and dynamically recoverable mechanism effectively bridges the gap between idealized laboratory conditions and the harsh, variable environments encountered in practical operation, addressing a critical bottleneck in the commercial viability of seawater electrolysis. The complex and dynamic interplay between the passivation layers and the operating environment, as revealed in this study, provides a solid foundation for future exploration. We anticipate that machine learning algorithms will play an increasingly important role in this endeavor. By training on the fundamental principles uncovered here, machine learning models can rapidly screen and optimize a wide range of material compositions and structures using predefined algorithms and key performance parameters, thereby accelerating the discovery of next-generation catalysts with enhanced self-healing capabilities. This synergistic approach, combining mechanistic insights with data-driven design, offers a clear and efficient pathway toward the practical realization of sustainable hydrogen production from seawater.
Authors contribution
Wang L: Data analysis, investigation, validation, writing-original draft, writing-review & editing.
Yuan ZY: Conceptualization, project administration, supervision, validation, writing-review & editing.
All authors have given approval to the final version of the manuscript.
Conflicts of interest
Zhong-Yong Yuan is the Editor-in-Chief of Smart Materials and Devices. Another author has no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (22179065), and the Ph.D. Candidate Research Innovation Fund of NKU School of Materials Science and Engineering.
Copyright
© The Author(s) 2025.
References
-
1. Wang HY, Wang L, Ren JT, Tian W, Sun M, Feng Y, et al. Taking advantage of potential coincidence region: advanced self-activated/propelled hydrazine-assisted alkaline seawater electrolysis and Zn-Hydrazine battery. ACS Nano. 2023;17(11):10965-10975.[DOI]
-
2. Zhang J, Fu X, Kwon S, Chen K, Liu X, Yang J, et al. Tantalum-stabilized ruthenium oxide electrocatalysts for industrial water electrolysis. Science. 2025;(6729):48-55.[DOI]
-
3. Logeshwaran N, Vijayapradeep S, Kim AR, Sampath P, Ramakrishnan S, Poudel M, et al. Study of engineering electronic structure modulated non-noble metal oxides for scaled-up alkaline blend seawater splitting. J Energy Chem. 2023;86:167-179.[DOI]
-
4. Zhu S, Qin X, Xiao F, Yang S, Xu Y, Tan Z, et al. The role of ruthenium in improving the kinetics of hydrogen oxidation and evolution reactions of platinum. Nat Catal. 2021;4(8):711-718.[DOI]
-
5. Yu PC, Zhang XL, Zhang TY, Tao XY, Yang Y, Wang YH, et al. Nitrogen-mediated promotion of cobalt-based oxygen evolution catalyst for practical anion-exchange membrane electrolysis. J Am Chem Soc. 2024;146(29):20379-20390.[DOI]
-
6. Kim HJ, Kumar RS, Tamilarasi S, Vijayapradeep S, Kwak HB, Yoo DJ. Elevated electrocatalytic activity of high-efficiency urea induces water electrolysis via a ruthenium nickel oxynitride electrocatalyst. Chem Eng J. 2024;489:151003.[DOI]
-
7. Zhang X, Lu G, Ning X, Wang C. Boron substitution enhanced activity of BxGa1-xAs/GaAs photocatalyst for water splitting. Appl Catal B Environ. 2022;300:120690.[DOI]
-
8. Xie H, Zhao Z, Liu T, Wu Y, Lan C, Jiang W, et al. A membrane-based seawater electrolyser for hydrogen generation. Nature. 2022;612(7941):673-678.[DOI]
-
9. Wang HY, Weng CC, Ren JT, Yuan ZY. An overview and recent advances in electrocatalysts for direct seawater splitting. Front Chem Sci Eng. 2021;15:1408-1426.[DOI]
-
10. Chen L. Electrocatalytic seawater splitting from direct electrolysis to hybrid electrolysis: challenges and opportunities. Mater Today. 2025;86:282-316.[DOI]
-
11. Guo J, Zheng Y, Hu Z, Zheng C, Mao J, Du K, et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat Energy. 2023;8:264-272.[DOI]
-
12. Wang HY, Ren JT, Wang L, Sun ML, Yang HM, Lv XW, et al. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis. J Energy Chem. 2022;75:66-73.[DOI]
-
13. Kang X, Yang F, Zhang Z, Liu H, Ge S, Hu S, et al. A corrosion-resistant RuMoNi catalyst for efficient and long-lasting seawater oxidation and anion exchange membrane electrolyzer. Nat Commun. 2023;14(1):3607.[DOI]
-
14. Yang F, Luo Y, Yu Q, Zhang Z, Zhang S, Liu Z, et al. A durable and efficient electrocatalyst for saline water splitting with current density exceeding 2000 mA cm-2. Adv Funct Mater. 2021;31(21):2010367.[DOI]
-
15. Sha Q, Wang S, Yan L, Feng Y, Zhang Z, Li S, et al. 10,000-h-stable intermittent alkaline seawater electrolysis. Nature. 2025;639:360-367.[DOI]
-
16. Liang J, Li Z, Zhang M, Wang H, Cai Z, Luo Y, et al. Amorphous phosphates tailor local proton supply for alkaline hydrogen evolution electrocatalysis. Adv Mater. 2025;37(33):2503660.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




