Abstract
Significant progress in clinical care has extended human life expectancy to unprecedented levels. However, this trend has been parallelled by a rise in years lived with poor health, posing profound challenges not only to individual quality of life, but also to substantial medical and socioeconomic burdens at the population level. This underscores the urgent need for strategies that extend healthspan alongside lifespan. In this regard, nicotinamide adenine dinucleotide (NAD+) has emerged as a central metabolic cofactor and signaling molecule that regulates processes fundamental to health and longevity, including energy metabolism, mitochondrial function, inflammation, and DNA repair. Importantly, intracellular NAD+ levels decline with age across multiple tissues and organ systems, and restoring NAD+ content has been shown to reinstate cellular and physiological function in various model systems. Among the strategies to augment NAD+, supplementation with its precursors, namely nicotinic acid/niacin, nicotinamide, nicotinamide riboside, and nicotinamide mononucleotide, represents the most practical and extensively studied approach. Over the past two decades, preclinical research and an increasing number of clinical trials have investigated the therapeutic potential of these precursors in preventing or reversing age-associated decline and pathologies. In this review, we synthesize recent clinical advances, critically evaluate the promise and limitations of NAD+ precursor supplementation, and discuss future directions for leveraging NAD+ metabolism to improve healthspan in a rapidly aging global population.
Keywords
1. Introduction
Aging is one of, if not the leading risk factor for the development of chronic disorders, including cardiovascular, metabolic, and neurodegenerative diseases, and musculoskeletal impairments[1]. Over the past decade, substantial progress has been made in understanding the fundamental mechanisms of aging and their contribution to age-related functional decline[2], suggesting that slowing the aging process itself may delay the onset and progression of late-life chronic diseases[3]. There is a growing recognition that, unlike chronological aging, the mere passage of time, biological aging, which reflects the rate at which cells and organs loose function, is a modifiable risk factor. Ample preclinical evidence demonstrating that targeting the hallmarks of aging can slow age-associated deterioration has fueled interest in human trials aimed at evaluating potential geroprotective therapies[3,4]. Optimizing these therapeutic approaches for clinical use could provide safe and effective strategies to improve quality of life and reduce morbidity in older individuals.
Emerging experimental and epidemiological studies indicate that aging is associated with a gradual decline in nicotinamide adenine dinucleotide (NAD+)[5-7], a central molecule in energy metabolism and a pleiotropic coenzyme involved in, but not limited to, cell signaling, mitochondrial function, post-translational protein modifications, epigenetic regulation, and autophagy. These cellular processes and functions are critical for maintaining tissue and metabolic homeostasis and for healthy aging[8]. Recent findings have transformed our understanding of NAD+ metabolism and guided research aimed at uncovering why aging drives NAD+ decline in certain tissues and how non-uniform NAD+ decline impacts physiological function in health and disease.
Aberrant NAD+ metabolism in general, and declines in NAD+ levels in particular, are implicated in the pathogenesis of multiple age-related disorders, ranging from cardiovascular and metabolic diseases[5,9,10], cognitive decline and cerebrovascular disorders[11] to muscular impairments[12]. Conversely, therapeutic replenishment of NAD+ levels through supplementation with its precursors reduces chronic low-grade inflammation, reactivates autophagy and mitochondrial biogenesis, and enhances oxidative metabolism in various cell types in both humans and rodents with age-related pathologies[8].
The accumulating experimental evidence supporting the broad health-improving effects of NAD+-boosting agents, which can attenuate age-related functional decline and increase healthspan in various experimental models of aging, has spurred scientific and public interest in translating NAD+-based therapies into clinical practice[8,9,13,14]. Despite extensive preclinical evidence demonstrating the benefits of NAD+, clinical studies still lag behind. To date, a limited number of trials have been completed, and many of these focused on safety, tolerability, and the ability of NAD+ precursors to increase NAD+ bioavailability. Moreover, only a few trials have consistently reported an age-related decline in NAD+ levels in humans. Nonetheless, completed clinical studies investigating the therapeutic potential of NAD+ precursors in age-related diseases highlight that we are only beginning to understand both the challenges and opportunities of translating promising preclinical findings from anti-aging studies into meaningful health benefits in humans.
In this review, we summarize and critically assess clinical trials completed over the past decade investigating NAD+ precursors in the context of aging and age-related disorders. We outline future directions and challenges to advance research aimed at defining the therapeutic value of NAD+ precursors and supporting their implementation in clinical practice to promote healthy aging. For a comprehensive overview of the physiological functions of NAD+, intracellular NAD+ metabolism, including NAD+ biosynthesis and consumption, subcellular compartmentalization of NAD+, inhibitors of NAD+-consuming enzymes, as well as NAD+ bioavailability, we refer readers to other relevant in-depth reviews[5,8,13,15].
2. Human Trials Exploring NAD+ Precursors Supplementation in Aging and Age-Related Disorders
NAD+ precursors are vitamin B3 derivatives and important dietary components found in various foods. The main dietary sources of NAD+ are nicotinamide (NAM) and nicotinic acid (NA), collectively referred to as preformed niacin, which is an essential micronutrient and often fortified in food staples (Figure 1), like rice, flour, cereals, bread, pasta and milk to improve their nutritional value and help prevent niacin deficiencies that can lead to pellagra[16]. Furthermore, NAD+ precursors such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) have been proposed as alternative, and arguably more effective[17], NAD+ enhancers to counteract metabolic and age-related functional decline[18]. Based on promising findings from rodent studies, which demonstrated that increasing intracellular NAD+ levels may help improve various human age-related diseases and comorbidities, clinical trials have primarily investigated the preventive and therapeutic benefits of oral supplementation with NAD+ precursors (Figure 2). The majority of NAD+-centered clinical trials have determined the safety, tolerability, and bioavailability of different naturally occurring NAD+ precursors in older individuals. Human studies consistently show that oral supplementation with various NAD+ precursors is generally safe and well tolerated, while only a minority of trials have reported mild side effects or negative outcomes. For example, supplementation with NR combined with pterostilbene, a sirtuin activator, was associated with minor gastrointestinal symptoms, nausea, and diarrhea in aged patients with acute kidney injury[19] as well as in otherwise healthy older adults[20]. Another study suggested that exposure to excessive niacin can provoke vascular inflammation and increase risk of cardiovascular disease[21], although these observations remain correlative and require further validation in future studies.
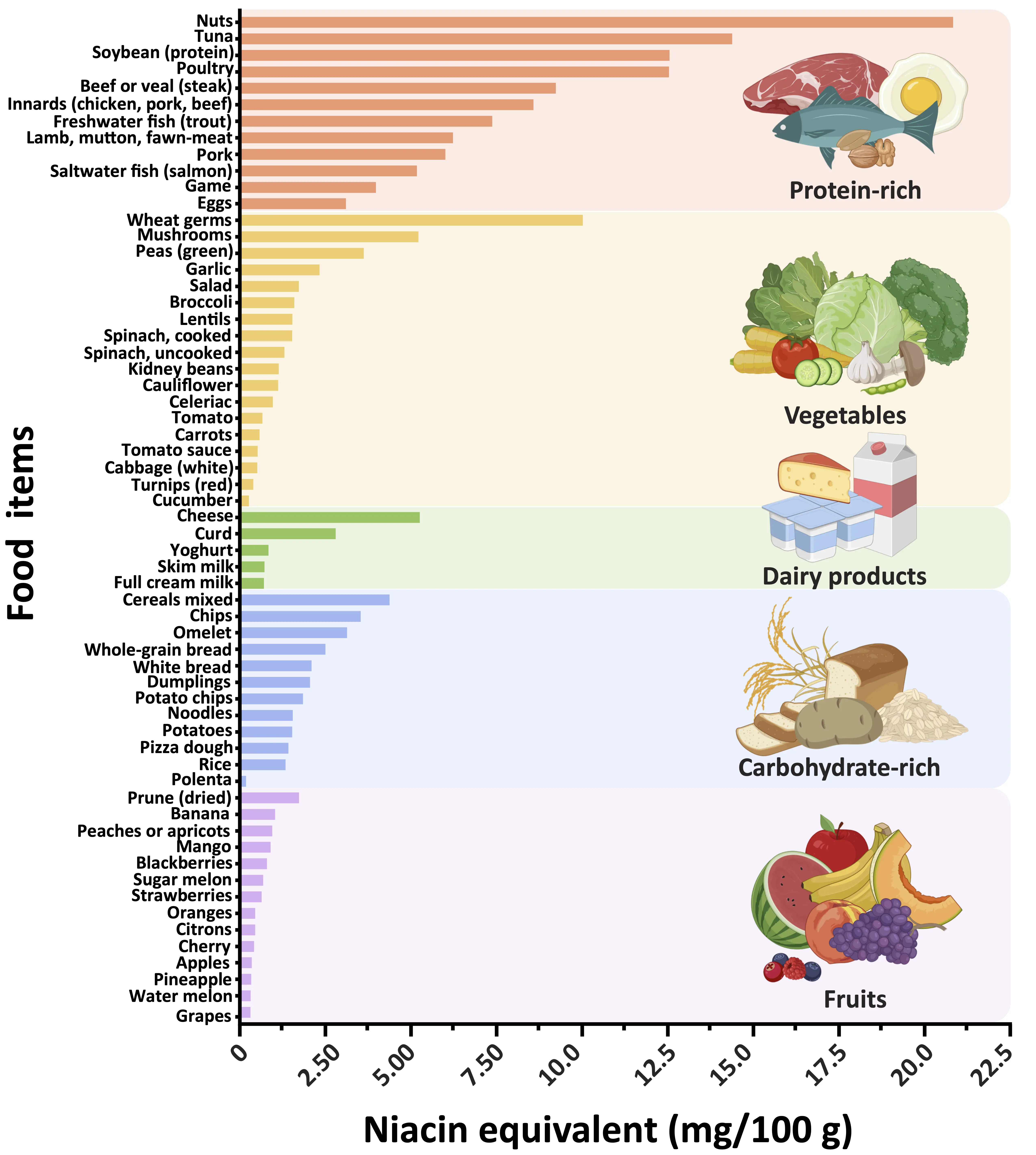
Figure 1. Classification of foods by category and their nutritional values for niacin equivalents. The graph depicts a range of selected foods, ranked based on their niacin equivalent content. The main dietary sources of NAD+ are nicotinamide and nicotinic acid, collectively referred to as preformed niacin. One milligram of niacin equivalents corresponds to either 1 mg of niacin or 60 mg of tryptophan. For similar food items (e.g., types of cheese), average values were calculated and grouped accordingly. Nutritional values for niacin equivalents were sourced from the German Nutrient Data Base (https://www.blsdb.de/; Federal Ministry of Food and Agriculture, Karlsruhe, Germany). Created in BioRender.com. NAD+: nicotinamide adenine dinucleotide.
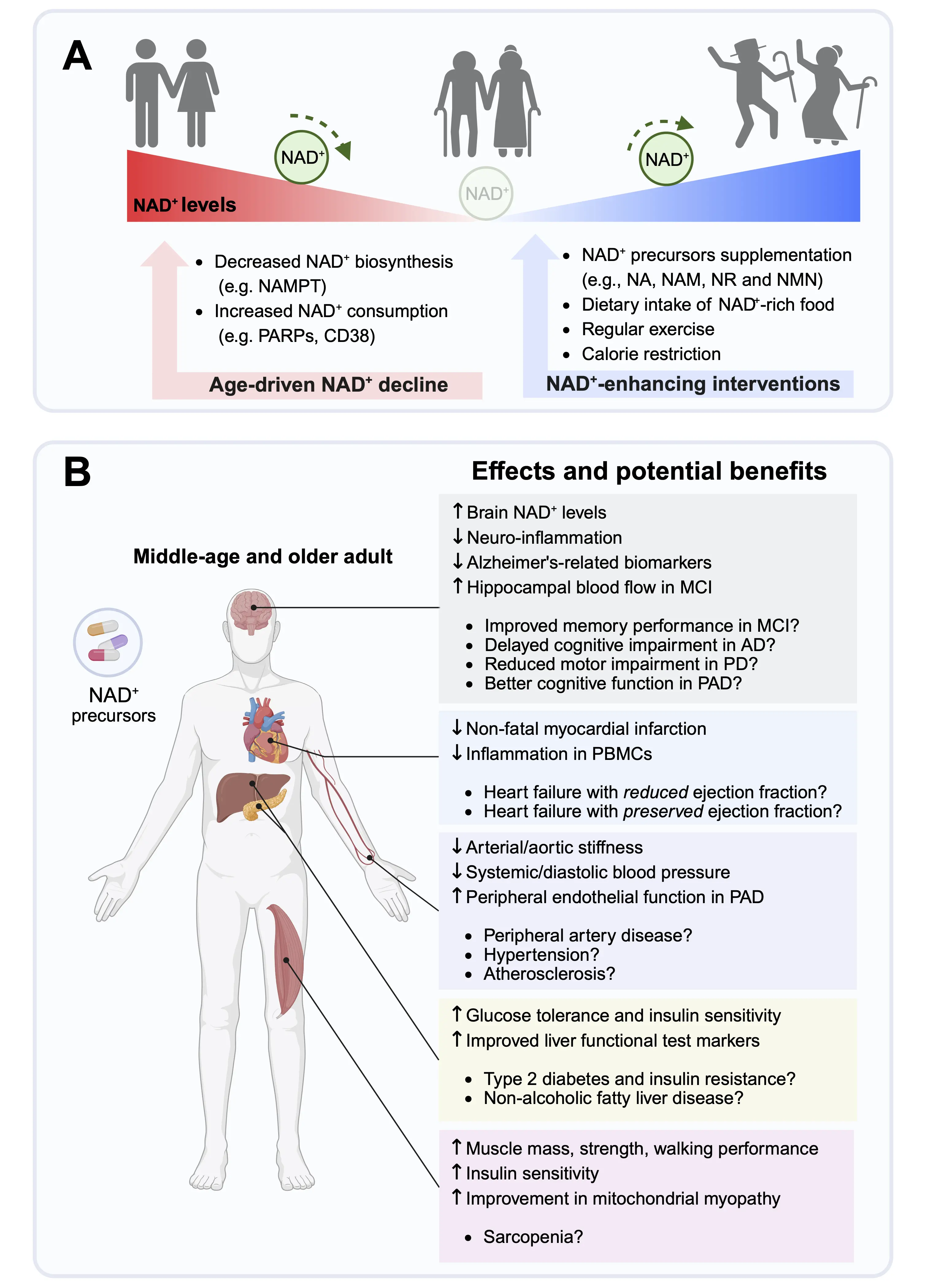
Figure 2. Clinical outcomes and potential benefits of NAD+ augmentation in age-associated disorders. (A) Aging is associated with a gradual decline of intracellular NAD+ levels due to reduced NAD+ biosynthesis, increased NAD+ consumption or both. NAMPT is the rate-limiting enzyme of the NAD+ salvage pathway and a key regulator of the intracellular NAD+ content. Major NAD+ consumers include PARPs, and cADPR synthase (e.g., CD38), and sirtuins. Potential interventions that increase cellular NAD+ content include NAD+ precursor supplementation, such as NA, NAM, NR, and NMN, increased intake of foods containing high levels of NAD+, regular physical exercise, and caloric restriction; (B) Effects and potential health benefits of NAD+ precursors supplementation in middle-aged and older adults. Arrows indicate effects that are up- or downregulated in response to NAD+ precursors supplementation, as demonstrated in clinical trials. Points indicate potential preventive and therapeutic benefits of NAD+ precursors to delay age-related diseases. Created in BioRender.com. NAD+: nicotinamide adenine dinucleotide; AD: Alzheimer’s disease; MCI: mild cognitive impairment; NA: nicotinic acid; NAM: nicotinamide; NAMPT: nicotinamide phosphoribosyltransferase; NMN: nicotinamide mononucleotide; NR: nicotinamide riboside; PBMCs: peripheral blood mononuclear cells; PD: Parkinson’s disease; PAD: peripheral artery disease; PARPs: poly(ADP-ribose) polymerases.
We performed a systematic search in the PubMed database for randomized clinical trials published between 2020 and 2025 using terms related to NAD+ precursors (e.g., nicotinamide riboside, nicotinamide mononucleotide, niacin, nicotinic acid, nicotinamide) in combination with aging or age-related diseases. In Table 1, we provide a comprehensive overview of recently completed and published trials testing the efficacy of natural NAD+ precursors against cardiac-, vascular-, cerebral-, metabolic-, and skeletomuscular-related endpoints in middle-aged and older adults.
| Disease/target population | Number and health status of participants | Precursor | Regimen/dose | Study findings | Side effects | References |
| Healthy | Healthy young- and old-aged adults, N = 24 | NR | 500 mg (single dose) | Increased NAD(P)H levels, decreased oxidative stress, and improved physical performance only in old individuals | Not observed | [51] |
| Healthy old-aged adults, N = 22-24 | 500 mg twice a day, 6 weeks | Increased serum NAD+ metabolome, decreased neurodegenerative markers in neuron-derived extracellular vesicles | Not observed | NCT02921659[70] | ||
| Healthy middle-aged men, N = 42 | NMN | 250 mg/day, 6 or 12 weeks | Trend towards improvements in gait speed and left grip strength | Safe | [112] | |
| Healthy old-aged men, N = 10 | 100/250/500 mg (single dose) | No adverse effects | Safe | [40] | ||
| Healthy middle age adults, N = 10 | 9 mmol/L (single intravenous dose) | Reduced blood triglyceride levels | Safe | [113] | ||
| Healthy middle- and old-aged adults, N = 66 | 250 mg/day, 60 days | Increased serum NAD+, helped to maintain healthy glycemic profile | Safe | NCT04228640[114] | ||
| Old adults with deteriorating sleep quality, fatigue, and impaired physical performance, N = 108 | 250 mg (morning or evening dose), 12 weeks | Improved physical performance and less fatigue specifically in the evening intervention group | Not observed | [55] | ||
| Cardiovascular disease | Old-aged patients with heart failure with reduced ejection fraction, N = 30 | NR | 1 g/day (twice daily), 12 weeks | Increased whole blood NAD+ levels, which correlated with increased respiration and decreased proinflammatory cytokine expression in peripheral immune cells. | Safe | NCT03423342[95] |
| Middle-aged and old adults with hypertension, N = 54 | 1 g/day NR with 3 days/week of supervised 30 mins walking exercise, 6 weeks | NR + exercise showed a trend towards greater nighttime blood pressure reduction | Safe | [64] | ||
| Old-aged patients with peripheral artery disease, N = 8 | 1 g/day NR, 4 weeks | Improvement in vascular and cognitive health | Not observed | NCT06534944[49] | ||
| Old-aged patients with lower extremity peripheral artery disease, N = 90 | 1 g/day NR alone and NR with resveratrol, 6 months | Improvement in 6-minute walk distance at 6-month follow-up | Not observed | NCT03743636[54] | ||
| Old-aged overweight or obese adults, N = 30 | β-NMN | 500 mg β-nicotinamide mononucleotide (MIB-626) (twice daily), 28 days | Decreased body weight, diastolic blood pressure and total cholesterol. | Safe | [42] | |
| Old-aged patients with heart failure with reduced ejection fraction, N = 12 | Niacin | Single dose of 1g (oral) Niacin | Decreased serum free fatty acids, no change in cardiac output. | Flushing and facial heat sensation | NCT04703361[42] | |
| Metabolic disorders | Old-aged obese and insulin resistance men, N = 40 | NR | 1 g (twice daily), 12 weeks | No change in skeletal muscle mitochondrial amount, morphology and respiration | Not observed | NCT02303483[56] |
| Post-menopausal women with prediabetes,overweight or obese, N = 25 | NMN | 250 mg/day, 10 weeks | Increased insulin-stimulated glucose disposal and skeletal muscle insulin signaling | Not observed | NCT03151239[65] | |
| Old adults with diabetes and impaired physical performance, N = 14 | 250 mg/day, 24 weeks | No improvement in grip strength and walking speed | Safe | [58] | ||
| Neurodegenerative diseases | Old-aged Parkinson’s disease patients, N = 47 | Niacin | 100-250 mg/day, 3-12 months | Trend to improved mood and motor functions | Mild flushing at 100 mg/day | [115,116] |
| Old-aged Parkinson’s disease patients, N = 30 | NR | 1 g/day, 30 days | Increased brain NAD+ metabolism, decreased neuronal inflammation. | Safe | NCT03816020[80] | |
| Old-aged mild cognitive impairment patients, N = 20 | Dose escalation to a final dose of 1 g/day over a 10-week study duration | Increased blood NAD+ levels, no change in cognitive function | Safe | NCT02942888[71] | ||
| Old-aged mild cognitive impairment or Alzheimer's disease patients, N = 40 | NAM | 1.5 g (twice daily), 48 weeks | Potentially slower CSF p-tau231 protein accumulation | Safe | NCT03061474[76] | |
| Chronic kidney disease | Old-aged chronic kidney disease patients, N = 25 | NR | NR (1 g/day) and Coenzyme Q10 (1.2 g/day), 6 weeks | Improved markers of systemic mitochondrial metabolism and blood lipid profile, No improvement in maximal oxygen consumption or total work efficiency | Not observed | NCT03579693[43] |
| Fatty Liver Disease | Middle-aged non-alcoholic fatty liver disease patients, N = 111 | NR | NRPT (nicotinamide riboside and pterostilbene) a recommended dose (NRPT 1x) and a double dose (NRPT 2x), 6 months | Improvement in liver health | Safe | NCT03513523[117] |
| Skeletomuscular disorder | Young- and old-aged patients with mitochondrial myopathy, N = 15 | Niacin | 750-1,000 mg/day, 10 months | Increase in skeletal muscle mitochondrial functions, muscle strength, and physical performance | Not observed | NCT03973203[53] |
| Old adults with experimental muscle injury, N = 32 | NR | 1 g/day NR and 200 mg/day PT, two weeks before injury and continued until 30 days after | No effect on muscle stem cell content and proliferation. No effect on muscle fiber area. | Safe | NCT03754842[118] | |
| COPD | Old-aged patients, N = 58 | NR | 2 g/day, 6 weeks | Significantly reduced sputum interleukin-8 levels. | Safe | NCT04990869[97] |
NAD+: nicotinamide adenine dinucleotide; NA: nicotinic acid; NAM: nicotinamide mononucleotide; NMN: nicotinamide mononucleotide; NR: nicotinamide riboside; COPD: chronic obstructive pulmonary disease; NRPT: nicotinamide riboside with pterostilbene.
2.1 NAD+ precursors against dyslipidemia to reduce cardiovascular disease risk
A major contributor to unhealthy aging is obesity and associated dyslipidemia, both of which have been growing exponentially over recent decades[22]. Obesity accelerates the aging process, while aging exacerbates the metabolic consequences of obesity, creating a vicious cycle that has contributed to a global surge in cardiovascular disease, currently affecting approximately 640 million people worldwide[22]. The causal relationship between obesity and cardiovascular aging has driven substantial interest in interventions aimed at improving lipid profiles to reduce obesity and mitigate the associated risk of cardiovascular disease and metabolic disorders in older adults.
Among the various NAD+ precursors, niacin, alone or in combination with statins, has received considerable attention for its potent lipid-lowering effects in this context. NA (commonly referred to as niacin) is the most extensively studied NAD+ precursor for reducing cardiovascular risk, not by increasing NAD+ itself, but through the management of dyslipidemia and related atherosclerosis. Its ability to lower circulating triglycerides and low-density lipoprotein (LDL) cholesterol while increasing high-density lipoprotein (HDL) cholesterol[23,24] has historically been associated with reduced mortality in humans[25]. However, recent large trials, including the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH)[26] and the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE)[27], demonstrated no additional reduction in cardiovascular risk when niacin was added to statin therapy, despite improved HDL and triglyceride levels. Both trials used sustained-release formulations to improve tolerability, as high dose NA frequently causes flushing, a cutaneous vasodilatory reaction primarily mediated by prostaglandin D2 release. Although extended-release forms of niacin reduce flushing, their lipid-lowering effect is associated with modest increases in adverse events, including disturbances in glucose control, gastrointestinal symptoms, and myopathy[27,28].
Ferrell et al. recently reported that increased levels of a terminal metabolite of excess niacin, N1-methyl-4-pyridone-3-carboxamide (4PY), were associated with endothelial inflammation and increased risk of major adverse cardiovascular events (MACE, a composite of strokes, myocardial infarctions, and death) in two cohorts involving more than 4,300 participants with stable cardiac disease[21]. Several interpretations could explain the correlational observations ascribed to niacin by Ferrell et al., who suggested that exposure to excessive niacin might provoke vascular inflammation and increase risk of cardiovascular disease[21]. First, higher 4PY levels could reflect independent nutritional and lifestyle habits, which predispose to MACE and/or simply serve as markers of chronic inflammatory vascular processes, suggesting that elevated 4PY levels are not causally responsible for the detrimental effects[29]. Intriguingly, niacin can inhibit vascular inflammation and protect against endothelial dysfunction independently of plasma lipid changes, particularly under low tryptophan conditions[30]. This anti-inflammatory effect appears to be mediated, at least in part, by direct interactions between tryptophan metabolites, such as NAM and NA, and the gut microbiome[31], especially when these metabolites are administered using a pH-targeted delayed-release formulation[32]. Secondly, overall diet quality, such as consumption of an unhealthy fast-food diet rather than micronutrient intake, may account for the observed association between 4PY and MACE[33]. Third, as 4PY is a terminal niacin metabolite designated for excretion, its increase could reflect impaired clearance rather than increased niacin intake, highlighting the need for metabolic flux assessments. Future research is required to clarify these assumptions and resolve the long-standing “niacin paradox”. In this context, Guyton and Boden recently proposed a thought-provoking “counterregulatory sympathetic stimulation” hypothesis[34]. Central to this theory is that niacin taken at bedtime may impair cardiovascular outcomes by substantially reducing non-esterified fatty acids. This can provoke fuel insufficiency during fasting and trigger a counterregulatory hormone response, including a catecholamine surge[35] and increased sympathetic nervous system activity, which may elevate the risk of myocardial infarction or sudden cardiac death, particularly in the morning[36,37]. Historically, niacin was taken with meals, whereas recent trials administered it at bedtime, possibly contributing to its lack of cardiovascular benefit when combined with statin therapy. Hence, future studies are needed to determine whether the timing of niacin administration (ie, bedtime vs. mealtime) is a critical factor affecting its efficacy and safety. Given that raising HDL with niacin does not further reduce cardiovascular risk in patients with low LDL, niacin is now primarily used as an alternative lipid-lowering agent for statin-intolerant patients.
Niacin is less potent at increasing NAD+ levels compared to other precursors, such as NR, NMN, and NAM[38]. Thus, the interest in human trials has shifted toward these NAD+-enhancing agents, particularly because they do not activate GPR109A receptors, which mediate the well-known flushing side effect of niacin. In this context, NR and NMN are the most commonly tested natural NAD+ precursors in clinical studies. Although the majority of these trials involved small numbers of participants, they consistently showed that various doses and durations of NR and NMN supplementation are well tolerated and increase whole blood NAD+ levels in healthy middle-aged and older adults[39,40]. Nevertheless, larger trials are needed to confirm the safety of these precursors, as the adverse effects of niacin became apparent in large-scale clinical trials enrolling thousands of participants and long-term follow-ups[27].
A recent meta-analysis of eight NMN-centered clinical trials conducted between 2021 and 2023 (dose range 250-2,000 mg/day for a duration of 14 days to 12 weeks), involving a total of 342 mainly non-diabetic and relatively healthy middle-age and older adults, reported no significant benefit of NMN on lipid profile or glucose control[41]. By contrast, oral supplementation of β-NMN (also known as MIB-626) in older adults with obesity increased circulating NAD+ levels, improved lipid parameters, and reduced diastolic blood pressure[42]. Similarly, NR supplementation combined with coenzyme Q10 improved markers of systemic mitochondrial metabolism and lipid profiles but did not enhance peak oxygen consumption or total work efficiency in middle-age patients with chronic kidney disease. Importantly, these findings were based on advanced lipidome analysis, and not traditional lipid panel values, such as LDL and HDL[43].
Like NR and NMN, NAM does not cause the flushing characteristic for NA/niacin. However, it has a more established safety profile, supported by phase 3 trials testing high doses over extended periods in both juvenile and older patients with type 1 diabetes and non-melanoma skin cancer, respectively, as demonstrated in the Oral Nicotinamide to Reduce Actinic Cancer (ONTARC)[44] and European Nicotinamide Diabetes Intervention Trial (ENDIT)[45] trials. Furthermore, NAM is disproportionately abundant among NAD+ precursors in both food and circulation[5], reinforcing its safety and suitability for human studies in other conditions. This finding opens a new perspective on the previously understudied therapeutic potential of NAM against cardiovascular disease. In support of this idea, a prospective community-based study enrolling an age- and sex-stratified random sample of 1,000 men and women (between the 4th and 7th decades) found that a diet enriched in NAM and NA was associated with lower blood pressure and reduced risk of overall and cardiac mortality over 20-year follow-up[46]. In another study, NAM depletion was associated with a more severe heart failure with preserved ejection fraction (HFpEF) phenotype and poorer prognosis. Specifically, older patients with HFpEF displaying a higher methyl-nicotinamide/nicotinamide ratio, indicative of reduced NAM bioavailability for NAD+ biosynthesis, had a significantly increased risk of adverse outcomes, a composite of cardiovascular death, heart failure hospitalization, urgent heart failure visits, or diuretic intensification[47,48].
Peripheral artery disease (PAD), characterized by systemic endothelial dysfunction, is associated with an increased risk for cerebral small vessel disease and vascular cognitive impairment in older adults. A pilot clinical study with an open-label design demonstrated that NR in older adults with PAD may lead to significant improvements in both macrovascular and microvascular endothelial function. Additionally, NR appeared to enhance cerebrovascular hemodynamics during cognitive tasks, resulting in modest gains in cognitive performance[49]. While these findings rely on surrogate endpoints, such as endothelial function and cognitive tests, they provide a rationale for future randomized controlled trials incorporating functional, anatomical, symptomatic, and clinical event-based outcomes to more accurately assess the benefits of NAD+ supplementation in mitigating age-related vascular and cognitive decline.
Taken together, these findings highlight both the promise and complexity of targeting NAD+ metabolism in cardiovascular aging. Niacin, the most extensively studied precursor, shows inconsistent clinical benefits, adverse effects, and mechanistic uncertainties, which have limited its broad clinical application. In contrast, newer precursors such as NR, NMN, and NAM exhibit more favorable safety profiles, reliable NAD+-boosting capacity, and early evidence of potential vascular and cardiometabolic benefits in older adults. Nevertheless, most clinical trials remain small and underpowered, underscoring the need for larger, long-term studies to determine whether NAD+ repletion can effectively reduce cardiovascular risk and chronic inflammation in aging populations with cardiovascular disease.
2.2 NAD+ precursors and exercise to improve skeletal muscle health in aging
Lower skeletal muscle mass and strength during aging can lead to progressive pathological muscle wasting and weakness, termed sarcopenia, which directly affects physical mobility and survival. A decline in cellular NAD+ levels has been associated with reduced mitochondrial oxidative capacity in patients with sarcopenia of different ethnicities between the ages of 65 and 79 years[12]. Moreover, skeletal muscle NAD+ abundance is reduced in physically impaired, but otherwise healthy, older adults[50], suggesting that increased energy expenditure during exercise, combined with compromised energy metabolism due to NAD+ insufficiency, may result in an inadequate exercise response. By contrast, exercise-trained older subjects exhibit NAD+ levels similar to those of younger individuals[50]. These findings support the ostensible link between normal cellular NAD+ content and a healthy muscle phenotype in humans. Importantly, these observations further suggest that maintaining physiological skeletal muscle NAD+ levels might not only prevent, but potentially reverse, age-associated physical weakness and even sarcopenia. In support of this idea, skeletal muscle biopsy studies in healthy elderly men showed that NR modulates muscle transcriptomes, enhances NAD+ metabolism (though not NAD+ abundance itself), improves redox status, and reduces inflammatory cytokine levels[43,51,52]. Similarly, niacin supplementation increased strength and muscle performance, an effect attributed to stimulated mitochondrial biogenesis and elevated mitochondrial mass in patients with mitochondrial myopathy[53]. In another study, six months of NR supplementation increased six-minute walk distance in older adults with PAD[54], whereas NMN supplementation for the same duration enhanced physical performance and reduced fatigue in older adults with low physical activity[55]. However, not all studies support the therapeutic potential of dietary NAD+ precursor supplementation against skeletomuscular diseases. For example, oral NR supplementation failed to increase muscle mitochondrial respiratory capacity, insulin sensitivity or muscle NAD+ content[52,56,57]. Similarly, NMN did not enhance physical performance in older adults with type 2 diabetes mellitus[58], and NR did not improve activity levels in patients with chronic kidney disease[43]. Additionally, no notable improvements in mitochondrial or skeletal muscle function were observed after supplementation with L-tryptophan, NA, and NAM in physically impaired, but otherwise healthy, older individuals[59]. A recent meta-analysis showed minimal potential of NR and NMN supplementation to improve sarcopenia-related conditions across diverse patient populations[60], further limiting the biomedical significance of these interventions. Nevertheless, it is important to note that these studies used relatively low oral doses of precursors, which may be suboptimal for effectively targeting skeletal muscle.
Aerobic exercise is widely regarded as the gold standard intervention for maintaining healthy skeletal muscle function at any stage of life. However, the effects of exercise have been shown to be variable in older adults[61,62]. For example, aerobic exercise can lower blood pressure, but the magnitude of this effect differs among hypertensive adults[63], with some older individuals showing resistance to exercise-induced reductions in systolic blood pressure[63]. This variability suggests that additional biological factors may influence individual responses to exercise. In this regard, it is tempting to speculate that reduced NAD+ abundance may be a critical biological factor contributing to the heterogeneity of physical function adaptations to exercise training in middle-aged and older adults. However, combining aerobic exercise with NR supplementation did not improve blood pressure control beyond that achieved with aerobic exercise alone in middle-aged and older adults with hypertension[64]. Given that this pilot study was of short duration (6 weeks) and involved a relatively small number of participants, larger and longer-term studies, incorporating alternative or more intensive exercise programs are warranted.
Taken together, current evidence indicates that NAD+ precursors supplementation induces a limited number of favorable molecular and metabolic adaptations in skeletal muscle, even in the absence of measurable increases in muscle NAD+ levels in biopsies from older adults. However, the translation of these biochemical changes into consistent, clinically meaningful gains in muscle strength or performance remains variable and appears to be influenced by age-associated comorbidities, baseline functional status, and disease context. Given that previous trials largely failed to elevate muscle NAD+ levels through oral precursors, intravenous delivery of NAD+ precursors, as reported mainly in case studies, may offer additional benefits by sustaining increases in muscle NAD+ content, although the evidence remains preliminary and speculative[64]. While exercise is the most powerful strategy to delay normal muscle aging and prevent sarcopenia, regular physical activity is often difficult to implement in older adults with comorbidities or poor baseline physical health. In this context, further studies are needed to test whether NAD+ precursor supplementation can enhance the biological responses to, and the efficacy of, regular exercise training in preventing or delaying age-related functional decline and comorbidities[62].
2.3 NAD+ precursors against the loss of metabolic control in aging
A decline in cellular NAD+ levels can contribute to the gradual loss of metabolic regulation and the onset of metabolic dysfunction during aging[6]. In turn, impaired metabolic control may exacerbate other aging processes, creating a self-perpetuating cycle that accelerates age-related decline. For instance, insulin sensitivity and glucose tolerance progressively decline with age, affecting multiple tissues, including—but not limited to—skeletal muscle, pancreas, liver, brain, and heart. Preclinical studies have shown that impaired NAD+ metabolism is closely linked to insulin resistance and glucose dysregulation. Reduced NAD+ levels and NAD+/NADH ratios, commonly observed in diabetes and obesity, contribute to mitochondrial dysfunction, increased accumulation of reactive oxygen species, and impaired glucose uptake[8]. Despite these promising experimental findings, clinical trials have produced mixed results. For example, oral NMN supplementation enhanced skeletal muscle insulin sensitivity in postmenopausal women with prediabetes who were overweight or obese[65]. However, aside from an approximately 20% increase in insulin sensitivity, NMN had no significant effects on body composition, liver/adipose fat, basal glucose and insulin levels, or other basal metabolic indicators, limiting the generalizability of these findings. In another study, NR supplementation modulated acetyl-carnitine metabolism in skeletal muscle and induced minor changes in body composition and sleeping metabolic rate in healthy overweight or obese men and women[57]. However, not all studies support the therapeutic potential of dietary NR supplementation. For example, NR supplementation did not improve insulin sensitivity or whole-body glucose metabolism in obese men with type 2 diabetes mellitus[66]. Similarly, short-term β-NMN supplementation did not change glucose metabolism or insulin sensitivity in middle-aged and older adults[42]. Furthermore, niacin showed no effect on fasting blood glucose or insulin levels in non-diabetic individuals[67]. Contrarily, an in-depth analysis of the AIM-HIGH trial cohort revealed that extended-release niacin did not improve glucose control in participants with normal fasting glucose and even increased plasma glucose levels in subjects at higher risk for diabetes[68].
Collectively, the effects of NAD+ precursor supplementation on glucose and insulin homeostasis in humans remain largely uncertain. Further research is needed to examine whether the discrepancies between animal and human studies are attributable to variables such as the relatively lower doses used in humans compared to animals, as well as other trial-related factors, like the age, sex, treatment duration, dietary habits, and underlying health conditions of the study population. Addressing these aspects in future studies will be essential to clarify whether NAD+ repletion can effectively improve metabolic markers of glucose tolerance and insulin resistance in age-related metabolic diseases.
2.4 NAD+ precursors to improve cognitive decline and neurodegenerative diseases
The brain has high energy demands and relies heavily on mitochondrial oxidative metabolism, making it particularly vulnerable to NAD+ deficits[69]. A growing body of evidence indicates that NAD+ depletion in neurons commonly occurs during aging and is further exacerbated in major neurodegenerative disorders[11]. Conversely, augmenting brain NAD+ metabolism mitigates pathological features in animal models of neurological disorders. Inspired by rodent studies, several clinical trials have investigated the effects of NAD+-targeted interventions on cognitive function and prevalent neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). Oral NR supplementation has been shown to increase neuronal NAD+ levels and modulate biomarkers related to neurodegenerative pathology, including reductions in amyloid-beta and tau proteins in neuronally-derived extracellular vesicles in middle-aged and older healthy adults[70]. In another study, NR supplementation increased blood NAD+ levels, which was associated with elevated DNA methylation and concomitant reduction in epigenetic age[71], or increased perfusion of the left hippocampus in older adults with mild cognitive impairment[72]. However, these changes did not translate into measurable improvements in memory performance. Similarly, NR supplementation lowered concentrations of pTau217, an early marker of Alzheimer’s disease, but failed to improve cognition in older adults with subjective cognitive decline or mild cognitive impairment[73]. Along the same line, clinical trials with NAM showed limited efficacy. High dose oral supplementation of NAM (1,500 mg twice daily for 48 weeks) was safe and tolerable and elevated whole-blood NAM levels. However, NAM increase in cerebrospinal fluid (CSF) was detectable in only a subset of participants with early Alzheimer’s disease[74]. Notably, many individuals exhibited low NAM levels in CSF despite high methyl-NAM levels, indicating that NAM crosses the blood-brain barrier but is rapidly methylated, reducing its effective CNS concentration[75]. As a result, NAM did not significantly alter disease-relevant biomarkers, such as tau protein phosphorylation[76]. It is important to mention that overexpression of NAM N-methyl transferase (NNMT), the enzyme that methylates NAM and prevents CNS accumulation, is associated with metabolic disorders[77,78], and multiple neurodegenerative diseases[79]. These findings suggest that low NAM bioavailability due to rapid methylation limits its use as a potential intervention for Alzheimer’s disease. In this regard, future trials might consider exploring NAM treatment in combination with inhibitors of NNMT to reduce methylation and improve NAM bioavailability in individuals with mild cognitive impairment or early Alzheimer’s disease.
In contrast to NAM, oral NR supplementation increased NAD+ levels in both the brain and CSF in patients with Parkinson’s disease[80]. Specifically, the NADPARK trial demonstrated that NR supplementation was associated with transcriptional upregulation of pathways related to mitochondrial, lysosomal, and proteasomal function in blood cells and/or skeletal muscle. Furthermore, NR reduced inflammatory cytokine levels in both serum and CSF. Further investigation in larger clinical trials is warranted to determine whether NR could serve as an effective neuroprotective treatment for Parkinson’s disease[80]. ALS is a devastating neurodegenerative disease characterized by the progressive loss of spinal and cortical motor neurons, leading to muscle atrophy. Because NAD+ metabolism regulates numerous cellular processes involved in ALS, including energy metabolism, mitochondrial function, inflammation, and DNA repair, NAD+ precursors have emerged as promising therapeutic candidates[81]. The combination of NR and pterostilbene (EH301), two compounds that synergistically increase NAD+ levels, has been demonstrated to significantly improve the ALS Functional Rating Scale-Revised score, which assesses disease severity, including respiratory function and muscle strength, in middle-aged patients[82]. Treatment with EH301 also increased the skeletal muscle/fat weight ratio, collectively supporting its potential disease-modifying effects. Nevertheless, the efficacy of EH301 requires validation in larger patient cohorts.
In summary, animal studies consistently support many neuroprotective and cognitive benefits from NAD+ precursor supplementation, whereas clinical outcomes have been less robust so far[83]. Evidence from emerging clinical trials using NAD+ precursors supplementation show improvements in a limited number of neurodegenerative biomarkers. Factors such as NAD+ bioavailability, dosage, metabolism (including gut microbiota-mediated effects), and the specific forms of NAD+ precursors may affect efficacy. More rigorous, larger-scale clinical trials across different neurodegenerative diseases and stages are needed to determine whether enhancing intracellular NAD+ levels can translate into tangible neuroprotective outcomes in older adults.
2.5 Therapeutic potential of NAD+ precursors in age-associated cancers
Aging is one of the strongest risk factors for most cancers, and age-related NAD+ decline has been implicated in multiple oncogenic processes, including genomic instability, impaired DNA repair, altered redox homeostasis, and immune dysregulation[84,85]. NAD+ serves as a critical co-substrate for enzymes involved in DNA damage surveillance and repair, particularly poly(ADP-ribose) polymerases (PARPs) and sirtuins, which are central to maintaining genomic integrity and chromatin structure[85]. Preclinical studies in aged rodents have shown that NAD+ repletion can enhance DNA repair capacity, suppress pro-inflammatory signaling, and modulate tumorigenesis[86-88]. However, translating these findings into clinical benefit is complex due to the highly context-dependent role of NAD+ metabolism in cancer. While NAD+ boosting may support genome maintenance, mitochondrial function, and immune surveillance in normal tissues, many tumors heavily rely on NAD+ biosynthesis, particularly via upregulation of the rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT), to sustain their elevated metabolic and biosynthetic demands[85,89]. Elevated NAMPT expression has been reported across multiple tumor malignancies, including colorectal, breast, prostate, and hematologic cancers, and is often associated with aggressive tumor behavior and poor prognosis. This has prompted the development of NAMPT inhibitors as potential anticancer agents, aiming to selectively deplete NAD+ in tumor cells. Several first-generation NAMPT inhibitors (eg, FK866/APO866, GMX1777, CHS-828) have entered early-phase clinical trials in advanced solid and hematologic malignancies. While these agents achieved on-target NAD+ depletion, their clinical use was limited by dose-limiting hematologic toxicities, particularly thrombocytopenia, and modest monotherapy efficacy[90,91]. Newer NAMPT-inhibiting agents, such as OT-82, are currently in phase I/II trials in hematologic cancers, with the goal of improving therapeutic windows and enabling patient stratification[92].
Importantly, despite theoretical concerns, current randomized human studies do not support the notion that NAD+ precursor supplementation increases cancer risk. On the contrary, evidence from clinical chemoprevention trials suggests potential benefit. In the phase III ONTRAC trial, oral supplementation of NAM (500 mg administered twice daily) reduced the incidence of new non-melanoma skin cancers by 23% over 12 months in high-risk older adults[93]. In contrast, a subsequent trial in high-risk, immunosuppressed organ transplant recipients did not replicate this protective effect, likely reflecting the distinct biology of carcinogenesis under chronic immunosuppression[44]. More recently, a phase IIb trial combining NAM with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor therapy in non-small-cell lung cancer reported significant improvements in progression-free and overall survival in selected subgroups, attributed to NAM-mediated reactivation of the tumor suppressor gene RUNX3[94].
Taken together, there is currently no reliable evidence that NAD+ precursors can treat tumors or improve cancer recurrence or survival in humans, aside from their role in skin cancer prevention. Given the dual role of NAD+ in supporting genomic maintenance while potentially fueling tumor metabolism, future clinical trials should carefully stratify participants by cancer risk, tumor type, and metabolic phenotype, and include mechanistic biomarkers to distinguish protective from potentially tumor-promoting effects upon NAD+ precursor supplementation.
2.6 NAD+ precursor supplementation in the regulation of immune function
Age-related declines in NAD+ metabolism impair immune cell function, driving chronic inflammation, a key hallmark of aging and age-associated diseases. Emerging clinical studies indicate that NAD+ precursor supplementation can modulate systemic immune function and inflammatory pathways in aging, although human trials testing immune-specific functional endpoints are surprisingly limited. For example, NR was shown to reduce systemic inflammation, partly by suppressing the pro-inflammatory activation of peripheral blood mononuclear cells in male patients with heart failure[95,96]. Mechanistically, NR-mediated NAD+ repletion lowered mitochondrial reactive oxygen species production, thereby attenuating NLRP3 inflammasome-caspase-1 activation and the secretion of IL-1β and IL-18[96]. In healthy older men, NR supplementation also decreased circulating inflammatory cytokines, including IL-6, IL-5, IL-2, and TNF-a, and reduced inflammatory gene expression in skeletal muscle[52]. Consistently, NR supplementation lowered airway inflammation in older patients with chronic obstructive pulmonary disease[97].
Interestingly, a study comparing NMN, NR, and NAM with the novel NAD+ precursor, dihydronicotinamide riboside (NRH) demonstrated that only NRH markedly increased NAD+ levels in both bone marrow-derived and THP-1 macrophages, a human monocytic cell line[98]. Notably, NRH supplementation also upregulated gene expression of several cytokines and chemokines, indicating that NAD+ elevation can promote inflammation in resting macrophages[98]. Nevertheless, further studies are warranted to elucidate the complex interplay between NAD+, its metabolites and precursors, and the enzymes that regulate them, in controlling immune function and inflammatory diseases.
Collectively, these data suggest that NAD+ precursors are capable of modulating immune cell signaling, whereas clinical confirmation of immunological benefits, particularly in aging, is still lacking. Future progress in this area will depend on incorporating immune-specific endpoints in clinical studies and focusing on age-related immune dynamics and relevant immune cell populations. Such multi-omic longitudinal analysis of the healthy human peripheral immune system may help to comprehensively evaluate the potential of NAD+ precursors in mitigating inflammatory responses as well as inflammation-associated pathologies.
3. Future Perspectives and Concluding Remarks
The ability of NAD+ precursors to modify the hallmarks of aging and mitigate age-related pathologies in animal models has spurred interest and efforts to translate these findings into human therapies. Clinical studies consistently show that oral administration of different NAD+ precursors is safe and tolerable at varied doses and treatment durations, and elevates NAD+ abundance and/or its metabolites, although to different extents, with no indications of serious side effects. However, it is important to note that large-scale and long-term trials, particularly for NR and NMN, remain scarce, limiting conclusions about sustained efficacy or rare adverse events. While certain trials report benefits on cardiovascular, metabolic, or physical outcomes, others show neutral effects, underscoring the need to clarify why NAD+ repletion works in some contexts but not others. Below, we discuss some of the areas of future research that are critical to gain a better understanding of systemic and tissue-specific NAD+ metabolism and for defining the therapeutic value of NAD+ precursors in geromedicine.
3.1 Uncertainties in NAD+ metabolism and its bioavailability
Studies in animal models have shown that NAD+ precursors exhibit differences in their absorption, conversion, and delivery to tissues[15,38]. For example, NA primarily supports hepatic NAD+ synthesis via the Preiss-Handler pathway, whereas NR and NMN are extracellularly converted to NAM prior to uptake, resulting in variable NAD+ bioavailability across tissues. However, no systematic head-to-head comparison has evaluated the efficiency with which each precursor restores NAD+ across different tissues in humans, as increases in whole blood NAD+ levels do not reflect organ- or compartment-specific NAD+ pools. It also remains unclear how different NAD+ precursors are absorbed, converted, and delivered to tissues in humans. In this context, an elegantly performed experimental study demonstrated that the decline in NAD+ with normal aging is relatively subtle and occurs despite maintained NAD+ production, likely due to increased consumption[99].
3.2 Age- and tissue-specific variability in NAD+ decline
Preclinical studies have demonstrated that age-related NAD+ decline is more pronounced in certain tissues, such as skeletal muscle, liver, adipose tissue, and brain, while being less evident in others, including kidney and the heart[7]. In humans, reductions in NAD+ with aging have been inconsistently observed in the brain, liver, skin, and skeletal muscle, whereas evidence for other tissues remains limited or inconclusive[15]. These findings indicate that NAD+ levels do not uniformly decline with age across all tissues, and that the variability may be also influenced, at least in part, by differences in measurement methods. Importantly, tissue-specific NAD+ turnover rates, enzymatic activity, metabolic demand, and baseline NAD+ status likely contribute to heterogeneous responses to NAD+ precursor supplementation.
3.3 Functional and translational impact of NAD+ precursor supplementation
A lack of head-to-head comparisons of the efficacy and pharmacokinetics of major NAD+ precursors limits a comprehensive understanding of systemic NAD+ metabolism in humans. This also hampers the ability to determine their benefits, potential risks, and to define the optimal precursor, dose, and duration for use in different patient populations. It is important to note that age-related NAD+ decline in humans is often relatively mild, making the therapeutic benefits of NAD+ precursors supplementation less pronounced than in animal models with engineered deficiencies in NAD+ metabolism. In addition, human evidence that NAD+ precursor supplementation can modulate immune and inflammatory pathways in older adults remains sparse. Although supplementation reliably increases NAD+ (or related metabolites) in blood and peripheral immune cells and, in some studies, has been associated with reduced pro-inflammatory signatures, translation of these molecular and cellular effects into clinically meaningful immune benefits in aging has not yet been demonstrated.
3.4 NAD+ precursor delivery and microbiome influence
Several practical aspects of NAD+ precursor supplementation need to be addressed, such as the optimal delivery method. For instance, slow-release NAM capsules have been designed to release their contents in the lower small intestine and colon rather than the stomach, ensuring systemic NAM availability while targeting distal regions of the intestinal tract, including the gut microbiota[32]. This ileocolonic delivery appears to provide high mucosal exposure without significantly altering the known side-effect profile and may modulate gut microbial metabolic activity, potentially reducing interindividual variability in NAD+ precursor metabolism and absorption. Oral bioavailability differences among NAD+ precursors, such as NR, likely depend not only on intestinal permeability but also on the gut microbiome. Indeed, emerging evidence suggests that gut microbes significantly modulate NAD+ precursor metabolism, potentially shaping individual responses. Specifically, bacterial-mediated deamidation contributes substantially to the NAD+-enhancing effects of oral NR and NAM supplementation in several tissues, highlighting the interplay between circulating host micronutrients and the gut microbiota[100,101]. Furthermore, NAM salvage genes nadV and pncA, present in distinct bacterial species, appear to have spread across the tree of life via horizontal gene transfer[102]. Recent advances in biochemical, genetic, and genomic analyses allow predictions of the precursors and pathways utilized by a given microorganism. Combining these analyses with machine learning algorithms can overcome challenges in understanding the regulation of these pathways. Future clinical trials and mechanistic studies are needed to compare systemic versus gut-targeted delivery routes, determine their relative clinical efficacy, and elucidate the precise mechanisms underlying their effects.
3.5 Methodological and trial limitations
Other practical issues include the standardization of sample collection procedures, analytical methods, and reliable biomarkers of NAD+ metabolism. Substantial progress has been made in improving the quantitation of NAD+ precursors and metabolites. For example, the development of NAD+ biosensors has advanced our understanding of how NAD+ levels are regulated both at the cellular and systems level. However, most clinical studies have used assays that measured steady-state NAD+ levels and related metabolites in body fluids and tissues, thereby providing an incomplete picture of the efficacy of NAD+ precursors in enhancing NAD+ bioavailability. Therefore, human studies employing isotope tracing are needed to accurately determine how NAD+ precursors modulate NAD+ flux, which more precisely reflects the dynamic balance between NAD+ synthesis and consumption[99,103-105]. Developing advanced methodologies for real-time NAD+ flux quantification and longitudinal of NAD+ profiling in easily accessible human tissues, such as skeletal muscle, will be crucial to determine whether and how aging alters tissue-specific NAD+ metabolism and why different tissues and organs exhibit variable responsiveness to NAD+ precursor supplementation. Early studies often used suboptimal dosing, short durations, or insensitive endpoints, limiting conclusions on efficacy of NAD+ precursor supplementation. Greater resources should be allocated to conducting longer and adequately powered randomized controlled human trials, involving both young and aging populations. Considering careful dose adaptation from rodent to human studies and assessing sex-specific effects of NAD+ precursor supplementation are necessary to determine whether commonly used classic NAD+ precursors or next-generation NAD+-enhancing compounds, such as NRH[98,106], reduced form of NMN (NMNH)[107], MIB-626 (a microcrystalline β-NMN formulation)[108], or a combination of nicotinamide with D-ribose[109], alone or in combination, can improve clinical outcomes. It will be also valuable to directly compare the efficacy of pharmacologically-elevated NAD+ levels with lifestyle modifications capable of increasing NAD+, such as regular exercise and caloric restriction[110,111], to ensure that patients can achieve maximal clinical benefit. Resolving these issues will set the basis for future directions in revealing the role of NAD+ during aging in humans. This knowledge will guide the design of future NAD+-based therapeutic interventions to delay aging and prevent age-related diseases.
Authors contribution
Khatri S, Sedej S: Conception and drafting of the manuscript, literature review, figures preparation.
Abdellatif M: Conception of the manuscript.
All authors participated in the critical revision, editing, and approval of the final manuscript.
Conflicts of interest
Mahmoud Abdellatif and Simon Sedej hold a patent related to the cardiometabolic effects of nicotinamide. Subhash Khatri reports no conflicts of interest. Simon Sedej and Mahmoud Abdellatif are Editorial Board Members of Geromedicine.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
The authors are grateful to the Austrian Science Fund (FWF) for the Excellence Cluster (10.55776/COE14), and acknowledge funding received from the Medical University of Graz (Excellence Cluster MetAGE and Flagship Project VASC-HEALTH), and BioTechMed-Graz (Flagship Project INTERACD+ to Simon Sedej, and Young Researcher Group to Abdellatif M). Mahmoud Abdellatif acknowledges additional funding received from the FWF (DOI: 10.55776/P34926) as well as (DOI: 10.55776/ I6931) under the umbrella of the Partnership Fostering a European Research Area for Health (ERA4Health; GA N 101095426 of the EU Horizon Europe Research and Innovation Program).
Copyright
© The Author(s) 2025.
References
-
1. Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, et al. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Sig Transduct Target Ther. 2022;7(1):391.[DOI]
-
2. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-78.[DOI]
-
3. Guarente L, Sinclair DA, Kroemer G. Human trials exploring anti-aging medicines. Cell Metab. 2024;36(2):354-76.[DOI]
-
4. Kroemer G, Maier AB, Cuervo AM, Gladyshev VN, Ferrucci L, Gorbunova V, et al. From geroscience to precision geromedicine: Understanding and managing aging. Cell. 2025;188(8):2043-2062.[DOI]
-
5. Abdellatif M, Sedej S, Kroemer G. NAD+ metabolism in cardiac health, aging, and disease. Circulation. 2021;144(22):1795-817.[DOI]
-
6. Chini CCS, Tarragó MG, Chini EN. NAD and the aging process: Role in life, death and everything in between. Mol Cell Endocrinol. 2017;455:62-74.[DOI]
-
7. Yoshino J, Baur JA, Imai SI. NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513-528.[DOI]
-
8. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119-1141.[DOI]
-
9. Abdellatif M, Baur JA. NAD+ metabolism and cardiometabolic health: The human evidence. Cardiovasc Res. 2021;117(9):e106-e1099.[DOI]
-
10. Abdellatif M, Bugger H, Kroemer G, Sedej S. NAD+ and vascular dysfunction: from mechanisms to therapeutic opportunities. J Lipid Atheroscler. 2022;11(2):111-132.[DOI]
-
11. Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 2019;30(4):630-655.[DOI]
-
12. Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun. 2019;10(1):5808.[DOI]
-
13. Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. 2020;2(1):9-31.[DOI]
-
14. Chini CCS, Zeidler JD, Kashyap S, Warner G, Chini EN. Evolving concepts in NAD+ metabolism. Cell Metab. 2021;33(6):1076-1087.[DOI]
-
15. Vinten KT, Trętowicz MM, Coskun E, van Weeghel M, Cantó C, Zapata-Pérez R, et al. NAD+ precursor supplementation in human ageing: Clinical evidence and challenges. Nat Metab. 2025;7(10):1974-1990.[DOI]
-
16. Park YK, Sempos CT, Barton CN, Vanderveen JE, Yetley EA. Effectiveness of food fortification in the United States: The case of pellagra. Am J Public Health. 2000;90(5):727-738.[DOI]
-
17. Yaku K, Palikhe S, Iqbal T, Hayat F, Watanabe Y, Fujisaka S, et al. Nicotinamide riboside and nicotinamide mononucleotide facilitate NAD+ synthesis via enterohepatic circulation. Sci Adv. 2025;11(12):eadr1538.[DOI]
-
18. Freeberg KA, Udovich CC, Martens CR, Seals DR, Craighead DH. Dietary supplementation with NAD+-boosting compounds in humans: current knowledge and future directions. J Gerontol A Biol Sci Med Sci. 2023;78(12):2435-2448.[DOI]
-
19. Simic P, Vela Parada XF, Parikh SM, Dellinger R, Guarente LP, Rhee EP. Nicotinamide riboside with pterostilbene (NRPT) increases NAD+ in patients with acute kidney injury (AKI): A randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol. 2020;21(1):342.[DOI]
-
20. Dellinger RW, Santos SR, Morris M, Evans M, Alminana D, Guarente L, et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: A randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;3:17.[DOI]
-
21. Ferrell M, Wang Z, Anderson JT, Li XS, Witkowski M, DiDonato JA, et al. A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat Med. 2024;30(2):424-434.[DOI]
-
22. Ruperez C, Madeo F, de Cabo R, Kroemer G, Abdellatif M. Obesity accelerates cardiovascular ageing. Eur Heart J. 2025;46(23):2161-2185.[DOI]
-
23. Carlson LA. Nicotinic acid: The broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258(2):94-114.[DOI]
-
24. Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54(2):558-559.[DOI]
-
25. Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: Long-term benefit with niacin. J Am Coll Cardiol. 1986;8(6):1245-1255.[DOI]
-
26. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255-2267.[DOI]
-
27. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203-212.[DOI]
-
28. Bays HE, Brinton EA, Triscari J, Chen E, Maccubbin D, MacLean AA, et al. Extended-release niacin/laropiprant significantly improves lipid levels in type 2 diabetes mellitus irrespective of baseline glycemic control. Vasc Health Risk Manag. 2015;11:165-172.[DOI]
-
29. Schreiber S, Waetzig GH, Laudes M, Rosenstiel P. Cardiovascular safety of vitamin B3 administration. Nat Med. 2024;30(9):2446-2447.[DOI]
-
30. Wu BJ, Yan L, Charlton F, Witting P, Barter PJ, Rye KA. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 2010;30(5):968-975.[DOI]
-
31. Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477-481.[DOI]
-
32. Schreiber S, Waetzig GH, López-Agudelo VA, Geisler C, Schlicht K, Franzenburg S, et al. Nicotinamide modulates gut microbial metabolic potential and accelerates recovery in mild-to-moderate COVID-19. Nat Metab. 2025;7(6):1136-1149.[DOI]
-
33. Lim GB. Metabolic product of excess niacin is linked to increased risk of cardiovascular events. Nat Rev Cardiol. 2024;21(5):280.[DOI]
-
34. Guyton JR, Boden WE. Niacin, food intake and cardiovascular effects. Nat Med. 2024;30(9):2444-2445.[DOI]
-
35. Watt MJ, Southgate RJ, Holmes AG, Febbraio MA. Suppression of plasma free fatty acids upregulates peroxisome proliferator-activated receptor (PPAR) alpha and delta and PPAR coactivator 1alpha in human skeletal muscle, but not lipid regulatory genes. J Mol Endocrinol. 2004;33(2):533-544.[DOI]
-
36. Muller JE, Tofler GH, Verrier RL. Sympathetic activity as the cause of the morning increase in cardiac events: A likely culprit, but the evidence remains circumstantial. Circulation. 1995;91(10):2508-2509.[DOI]
-
37. Willich SN, Linderer T, Wegscheider K, Leizorovicz A, Alamercery I, Schröder R. Increased morning incidence of myocardial infarction in the ISAM Study: absence with prior beta-adrenergic blockade. ISAM Study Group. Circulation. 1989;80(4):853-858.[DOI]
-
38. Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948.[DOI]
-
39. Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286.[DOI]
-
40. Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020;67(2):153-160.[DOI]
-
41. Chen F, Zhou D, Kong APS, Yim NT, Dai S, Chen YN, et al. Effects of Nicotinamide Mononucleotide on Glucose and Lipid Metabolism in Adults: A Systematic Review and Meta-analysis of Randomised Controlled Trials. Curr Diab Rep. 2024;25(1):4.[DOI]
-
42. Pencina KM, Valderrabano R, Wipper B, Orkaby AR, Reid KF, Storer T, et al. Nicotinamide adenine dinucleotide augmentation in overweight or obese middle-aged and older adults: A physiologic study. J Clin Endocrinol Metab. 2023;108(8):1968-1980.[DOI]
-
43. Ahmadi A, Begue G, Valencia AP, Norman JE, Lidgard B, Bennett BJ, et al. Randomized crossover clinical trial of coenzyme Q10 and nicotinamide riboside in chronic kidney disease. JCI Insight. 2023;8(11):e167274.[DOI]
-
44. Allen NC, Martin AJ, Snaidr VA, Eggins R, Chong AH, Fernandéz-Peñas P, et al. Nicotinamide for skin-cancer chemoprevention in transplant recipients. N Engl J Med. 2023;388(9):804-812.[DOI]
-
45. Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): A randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925-931.[DOI]
-
46. Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13(580):eabd7064.[DOI]
-
47. Abdellatif M, Vasques-Nóvoa F, Trummer-Herbst V, Durand S, Koser F, Islam M, et al. Autophagy is required for the therapeutic effects of the NAD+ precursor nicotinamide in obesity-related heart failure with preserved ejection fraction. Eur Heart J. 2025;46(19):1863-1866.[DOI]
-
48. Abdellatif M, Vasques-Nóvoa F, Ferreira JP, Sadoshima J, Diwan A, Linke WA, et al. NAD+ repletion restores cardioprotective autophagy and mitophagy in obesity-associated heart failure by suppressing excessive trophic signaling. Autophagy. 2025;21(10):2296-2298.[DOI]
-
49. Szarvas Z, Reyff ZA, Peterfi A, Pinto CB, Owens CD, Kaposzta Z, et al. Effects of NAD+ supplementation with oral nicotinamide riboside on vascular health and cognitive function in older adults with peripheral artery disease: Results from a pilot 4-week open-label clinical trial. J Pharmacol Exp Ther. 2025;392(7):103607.[DOI]
-
50. Janssens GE, Grevendonk L, Perez RZ, Schomakers BV, de Vogel-van den Bosch J, Geurts JMW, et al. Healthy aging and muscle function are positively associated with NAD+ abundance in humans. Nat Aging. 2022;2(3):254-263.[DOI]
-
51. Dolopikou CF, Kourtzidis IA, Margaritelis NV, Vrabas IS, Koidou I, Kyparos A, et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur J Nutr. 2020;59(2):505-515.[DOI]
-
52. Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717-1728.[DOI]
-
53. Pirinen E, Auranen M, Khan NA, Brilhante V, Urho N, Pessia A, et al. Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 2020;31(6):1078-1090.[DOI]
-
54. McDermott MM, Martens CR, Domanchuk KJ, Zhang D, Peek CB, Criqui MH, et al. Nicotinamide riboside for peripheral artery disease: The NICE randomized clinical trial. Nat Commun. 2024;15(1):5046.[DOI]
-
55. Kim M, Seol J, Sato T, Fukamizu Y, Sakurai T, Okura T. Effect of 12-week intake of nicotinamide mononucleotide on sleep quality, fatigue, and physical performance in older Japanese adults: a randomized, double-blind placebo-controlled study. Nutrients. 2022;14(4):755.[DOI]
-
56. Dollerup OL, Chubanava S, Agerholm M, Søndergård SD, Altıntaş A, Møller AB, et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J Physiol. 2020;598(4):731-754.[DOI]
-
57. Remie CME, Roumans KHM, Moonen MPB, Connell NJ, Havekes B, Mevenkamp J, et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr. 2020;112(2):413-426.[DOI]
-
58. Akasaka H, Nakagami H, Sugimoto K, Yasunobe Y, Minami T, Fujimoto T, et al. Effects of nicotinamide mononucleotide on older patients with diabetes and impaired physical performance: A prospective, placebo-controlled, double-blind study. Geriatr Gerontol Int. 2023;23(1):38-43.[DOI]
-
59. Connell NJ, Grevendonk L, Fealy CE, Moonen-Kornips E, Bruls YMH, Schrauwen-Hinderling VB, et al. NAD+-precursor supplementation with L-tryptophan, nicotinic acid, and nicotinamide does not affect mitochondrial function or skeletal muscle function in physically compromised older adults. J Nutr. 2021;151(10):2917-231.[DOI]
-
60. Prokopidis K, Moriarty F, Bahat G, McLean J, Church DD, Patel HP. The effect of nicotinamide mononucleotide and riboside on skeletal muscle mass and function: A Systematic Review and Meta-Analysis. J Cachexia Sarcopenia Muscle. 2025;16(3):e13799.[DOI]
-
61. Chmelo EA, Crotts CI, Newman JC, Brinkley TE, Lyles MF, Leng X, et al. Heterogeneity of physical function responses to exercise training in older adults. J Am Geriatr Soc. 2015;63(3):462-469.[DOI]
-
62. Custodero C, Saini SK, Shin MJ, Jeon YK, Christou DD, McDermott MM, et al. Nicotinamide riboside—A missing piece in the puzzle of exercise therapy for older adults? Exp Gerontol. 2020;137:110972.[DOI]
-
63. Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapiro EP, et al. Effect of exercise on blood pressure in older persons: A randomized controlled trial. Arch Intern Med. 2005;165(7):756-762.[DOI]
-
64. Lin Y, Zeidan RS, Lapierre-Nguyen S, Costello HM, Anton SD, Buford TW, et al. Nicotinamide riboside combined with exercise to treat hypertension in middle-aged and older adults: A pilot randomized clinical trial. Geroscience. 2025;47(4):1-4.[DOI]
-
65. Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372(6547):1224-1229.[DOI]
-
66. Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343-353.[DOI]
-
67. Hu M, Yang YL, Masuda D, Yamashita S, Tomlinson B. Effect of extended-release niacin/laropiprant combination on plasma adiponectin and insulin resistance in Chinese patients with dyslipidaemia. Dis Markers. 2015;2015(1):154014.[DOI]
-
68. Goldberg RB, Bittner VA, Dunbar RL, Fleg JL, Grunberger G, Guyton JR, et al. Effects of extended-release niacin added to simvastatin/ezetimibe on glucose and insulin values in AIM-HIGH. Am J Med. 2016;129(7):753.[DOI]
-
69. Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U.S.A. 2015;112(9):2876-2881.[DOI]
-
70. Vreones M, Mustapic M, Moaddel R, Pucha KA, Lovett J, Seals DR, et al. Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell. 2023;22(1):e13754.[DOI]
-
71. Orr ME, Kotkowski E, Ramirez P, Bair-Kelps D, Liu Q, Brenner C, et al. A randomized placebo-controlled trial of nicotinamide riboside in older adults with mild cognitive impairment. Geroscience. 2024;46:665-682.[DOI]
-
72. Martens CR, Decker KP, DeConne TM, Sanjana F, Horvat F, Rizzi NA, et al. A phase-II randomized controlled pilot and feasibility study of nicotinamide riboside supplementation in older adults with amnestic mild cognitive impairment. Alzheimer’s Association International Conference; Jul 27-31, 2025; Toronto, Canada. 2025.
-
73. Wu CY, Kupferschmid AC, Chen L, McManus AJ, Kivisäkk P, Galler JA, et al. Cognitive and Alzheimer’s disease biomarker effects of oral nicotinamide riboside (NR) supplementation in older adults with subjective cognitive decline and mild cognitive impairment. Alzheimer's Dement: Transl Res Clin Interv. 2025;11(1):e70023.[DOI]
-
74. Ketron GL, Grun F, Grill JD, Feldman HH, Rissman RA, Brewer GJ. Pharmacokinetic and pharmacodynamic assessment of oral nicotinamide in the NEAT clinical trial for early Alzheimer's disease. Alz Res Therapy. 2025;17(1):59.[DOI]
-
75. Spector R. Niacinamide transport through the blood-brain barrier. Neurochem Res. 1987;12(1):27-31.[DOI]
-
76. Grill JD, Tam S, Thai G, Vides B, Pierce AL, Green K, et al. Phase 2A Proof-of-Concept Double-Blind, randomized, Placebo-Controlled trial of nicotinamide in early alzheimer disease. Neurology. 2025;104(1):e210152.[DOI]
-
77. Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508(7495):258-262.[DOI]
-
78. Hong S, Moreno-Navarrete JM, Wei X, Kikukawa Y, Tzameli I, Prasad D, et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat Med. 2015;21(8):887-894.[DOI]
-
79. Kocinaj A, Chaudhury T, Uddin MS, Junaid RR, Ramsden DB, Hondhamuni G, et al. High expression of nicotinamide N-methyltransferase in patients with sporadic Alzheimer’s disease. Mol Neurobiol. 2021;58(4):1769-1781.[DOI]
-
80. Brakedal B, Dölle C, Riemer F, Ma Y, Nido GS, Skeie GO, et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson's disease. Cell Metab. 2022;34(3):396-407.[DOI]
-
81. Lundt S, Ding S. Potential therapeutic interventions targeting NAD+ metabolism for ALS. Cells. 2024;13(17):1509.[DOI]
-
82. de la Rubia JE, Drehmer E, Platero JL, Benlloch M, Caplliure-Llopis J, Villaron-Casales C, et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled human pilot study. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1-2):115-122.[DOI]
-
83. Campbell JM. Supplementation with NAD+ and its precursors to prevent cognitive decline across disease contexts. Nutrients. 2022;14(15):3231.[DOI]
-
84. Navas LE, Carnero A. NAD+ metabolism, stemness, the immune response, and cancer. Sig Transduct Target Ther. 2021;6(1):2.[DOI]
-
85. Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol. 2018;8:622.[DOI]
-
86. Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 2016;24(4):566-581.[DOI]
-
87. Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795-806.[DOI]
-
88. Wilk A, Hayat F, Cunningham R, Li J, Garavaglia S, Zamani L, et al. Extracellular NAD+ enhances PARP-dependent DNA repair capacity independently of CD73 activity. Sci Rep. 2020;10(1):651.[DOI]
-
89. Heske CM, Davis MI, Baumgart JT, Wilson K, Gormally MV, Chen L, et al. Matrix screen identifies synergistic combination of PARP inhibitors and nicotinamide phosphoribosyltransferase (NAMPT) inhibitors in Ewing sarcoma. Clin Cancer Res. 2017;23(23):7301-7311.[DOI]
-
90. Holen K, Saltz LB, Hollywood E, Burk K, Hanauske AR. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest New Drugs. 2008;26(1):45-51.[DOI]
-
91. Goldinger SM, Gobbi Bischof S, Fink-Puches R, Klemke CD, Dréno B, Bagot M, et al. Efficacy and safety of APO866 in patients with refractory or relapsed cutaneous T-cell lymphoma: A phase 2 clinical trial. JAMA Dermatol. 2016;152(7):837-839.[DOI]
-
92. Korotchkina L, Kazyulkin D, Komarov PG, Polinsky A, Andrianova EL, Joshi S, et al. OT-82, a novel anticancer drug candidate that targets the strong dependence of hematological malignancies on NAD biosynthesis. Leukemia. 2020;34(7):1828-1839.[DOI]
-
93. Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618-1626.[DOI]
-
94. Oh HJ, Bae SC, Oh IJ, Park CK, Jung KM, Kim DM, et al. Nicotinamide in combination with EGFR-TKIs for the treatment of stage IV lung adenocarcinoma with egfr mutations: A randomized double-blind (phase IIb) trial. Clin Cancer Res. 2024;30(8):1478-1487.[DOI]
-
95. Wang DD, Airhart SE, Zhou B, Shireman LM, Jiang S, Melendez Rodriguez C, et al. Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fraction. J Am Coll Cardiol Basic Trans Science. 2022;7(12):1183-1196.[DOI]
-
96. Zhou B, Wang DD, Qiu Y, Airhart S, Liu Y, Stempien-Otero A, et al. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest. 2020;130(11):6054-6063.[DOI]
-
97. Norheim KL, Ben Ezra M, Heckenbach I, Andreasson LM, Eriksen LL, Dyhre-Petersen N, et al. Effect of nicotinamide riboside on airway inflammation in COPD: A randomized, placebo-controlled trial. Nat Aging. 2024;4(12):1772-1781.[DOI]
-
98. Chini CCS, Peclat TR, Gomez LS, Zeidler JD, Warner GM, Kashyap S, et al. Dihydronicotinamide riboside is a potent NAD+ precursor promoting a pro-inflammatory phenotype in macrophages. Front Immunol. 2022;13:840246.[DOI]
-
99. McReynolds MR, Chellappa K, Chiles E, Jankowski C, Shen Y, Chen L, et al. NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Syst. 2021;12(12):1160-1172.[DOI]
-
100. Chellappa K, McReynolds MR, Lu W, Zeng X, Makarov M, Hayat F, et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 2022;34(12):1947-1959.[DOI]
-
101. Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020;31(3):564-579.[DOI]
-
102. Gazzaniga F, Stebbins R, Chang SZ, McPeek MA, Brenner C. Microbial NAD metabolism: lessons from comparative genomics. Microbiol Mol Biol Rev. 2009;73(3):529-541.[DOI]
-
103. Liu L, Su X, Quinn WJ, Hui S, Krukenberg K, Frederick DW, et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27(5):1067-1080.[DOI]
-
104. Song WS, Shen X, Du K, Ramirez CB, Park SH, Cao Y, et al. Nicotinic acid riboside maintains NAD+ homeostasis and ameliorates aging-associated NAD+ decline. Cell Metab. 2025;37(7):1616-1618.[DOI]
-
105. Migaud ME, Ziegler M, Baur JA. Regulation of and challenges in targeting NAD+ metabolism. Nat Rev Mol Cell Biol. 2024;25(10):822-8240.[DOI]
-
106. Yang Y, Mohammed FS, Zhang N, Sauve AA. Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J Biol Chem. 2019;294(23):9295-9307.[DOI]
-
107. Zapata-Pérez R, Tammaro A, Schomakers BV, Scantlebery AML, Denis S, Elfrink HL, et al. Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice. Faseb j. 2021;35(4):e21456.[DOI]
-
108. Pencina KM, Lavu S, Dos Santos M, Beleva YM, Cheng M, Livingston D, et al. MIB-626, an oral formulation of a microcrystalline unique polymorph of β-nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle-aged and older adults. J Gerontol A Biol Sci Med Sci. 2023;78(1):90-96.[DOI]
-
109. Xue Y, Shamp T, Nagana Gowda GA, Crabtree M, Bagchi D, Raftery D. A combination of nicotinamide and D-ribose (RiaGev) is safe and effective to increase NAD+ metabolome in healthy middle-aged adults: A randomized, triple-blind, placebo-controlled, cross-over pilot clinical trial. Nutrients. 2022;14(11):2219.[DOI]
-
110. Voglhuber J, Ljubojevic-Holzer S, Abdellatif M, Sedej S. Targeting cardiovascular risk factors through dietary adaptations and caloric restriction mimetics. Front Nutr. 2021;8:758058.[DOI]
-
111. Fuerlinger A, Stockner A, Sedej S, Abdellatif M. Caloric restriction and its mimetics in heart failure with preserved ejection fraction: mechanisms and therapeutic potential. Cardiovasc Diabetol. 2025;24(1):21.[DOI]
-
112. Igarashi M, Nakagawa-Nagahama Y, Miura M, Kashiwabara K, Yaku K, Sawada M, et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging. 2022;8(1):5.[DOI]
-
113. Kimura S, Ichikawa M, Sugawara S, Katagiri T, Hirasawa Y, Ishikawa T, et al. Nicotinamide mononucleotide is safely metabolized and significantly reduces blood triglyceride levels in healthy individuals. Cureus. 2022;14(9):e28812.[DOI]
-
114. Huang H. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Front Aging. 2022;3:851698.[DOI]
-
115. Wakade C, Chong R, Seamon M, Purohit S, Giri B, Morgan JC. Low-dose niacin supplementation improves motor function in US Veterans with Parkinson’s disease: a single-center, randomized, placebo-controlled trial. Biomedicines. 2021;9(12):1881.[DOI]
-
116. Chong R, Wakade C, Seamon M, Giri B, Morgan J, Purohit S. Niacin enhancement for Parkinson’s disease: an effectiveness trial. Front Aging Neurosci. 2021;13:667032.[DOI]
-
117. Dellinger RW, Holmes HE, Hu-Seliger T, Butt RW, Harrison SA, Mozaffarian D, et al. Nicotinamide riboside and pterostilbene reduces markers of hepatic inflammation in NAFLD: A double-blind, placebo-controlled clinical trial. Hepatology. 2023;78(3):863-877.[DOI]
-
118. Jensen JB, Dollerup OL, Møller AB, Billeskov TB, Dalbram E, Chubanava S, et al. A randomized placebo-controlled trial of nicotinamide riboside and pterostilbene supplementation in experimental muscle injury in elderly individuals. JCI Insight. 2022;7(19):e158314.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




