Abstract
As the primary skin-contact interface in wearable electrocardiograph (ECG) devices, epidermal electrodes play a pivotal role in determining both signal quality and biocompatibility. With continuous advancements in materials science and structural engineering, next-generation flexible and stretchable bioelectrodes have emerged, enabling long-term ECG monitoring and offering superior signal-to-noise ratios compared to conventional clinical electrodes. Their performance in ensuring reliable signal acquisition and user comfort is primarily governed by key interfacial mechanical and electrical properties, including mechanical compliance (i.e., flexibility and stretchability), interfacial adhesion (i.e., conformability and adhesion strength), and electrical characteristics (i.e., contact impedance). In recent years, significant progress has been made in enhancing the signal acquisition capabilities of flexible and stretchable bioelectrodes by optimizing these critical interfacial attributes. This review highlights the latest advances in conformable epidermal electrodes, encompassing traditional wet electrodes, flexible dry electrodes, novel dry electrodes based on organic electrochemical transistors, and integrated wearable systems. We systematically examine strategies for improving skin-electrode interface performance in ECG monitoring. Finally, we discuss ongoing challenges and future directions to advance epidermal electrode technologies for next-generation wearable healthcare applications.
Keywords
1. Introduction
As one of the vital signs, electrophysiological signals provide key clinical clues to an individual's health status and play a crucial role in assessing various aspects of physical activity. The electrocardiograph (ECG) signal reflects the regular changes in heart activity and offers valuable information for predicting and diagnosing cardiovascular diseases such as arrhythmia and atrial fibrillation[1-3]. Efficient and accurate detection of ECG signals is highly significant for cardiology research, clinical diagnosis, treatment of heart disease, and exercise analysis[4-6]. To promptly understand heart conditions, continuous recording of ECG signals for real-time monitoring of physical health is essential. This continuous monitoring is important for the timely and accurate diagnosis of early or subtle cardiovascular diseases. Wearable ECG monitoring devices, characterized by lightweight design, continuous monitoring capability, and real-time data transmission, are important tools for achieving continuous ECG signal detection. Compared to traditional ECGs, wearable ECG devices enable prolonged tracking of cardiac activity with high comfort and portability. These devices hold great value for predicting and diagnosing cardiovascular diseases (e.g., atrial fibrillation, myocardial ischemia) by analyzing long-term trends. Additionally, they assist physicians in identifying high-risk patients, optimizing personalized treatment, and reducing the likelihood of acute cardiac events.
As a key component of wearable ECG devices, flexible bioelectrodes or electronic devices have revolutionized the acquisition of electrophysiological signals through their conformal skin interfaces[7,8]. Advances in flexible and stretchable electrode materials and interfacial designs have enabled robust yet soft contact between these devices and the skin, allowing continuous, long-term, and accurate sensing while maintaining skin compatibility[7]. Significant progress in the development of epidermal electrodes and electronic devices has been achieved in recent years by improving interfacial adhesiveness, skin conformability, signal amplification, and mechanical flexibility and stretchability. Here, we introduce recent developments in soft, flexible, and stretchable skin-like bioelectrodes or electronic devices that conformally contact the epidermis specifically for ECG signal monitoring. Figure 1 illustrates examples of diverse flexible electrodes and electronic interfaces developed for ECG monitoring on the human body. For instance, robust soft metal electrodes are now used in the latest generation of commercial wearable ECG devices, replacing traditional rigid metal disc electrodes. Regarding traditional wet electrodes, recent efforts have focused on novel adhesive gel electrodes with good biocompatibility to improve skin friendliness and stability. The advances in flexible and stretchable dry electrodes are also detailed, particularly improvements in skin conformability and interfacial adhesiveness. The integration of bioelectrodes with flexible or stretchable electronics, commonly referred to as “electronic skin” or “epidermal electronics”, is a growing trend aimed at developing multifunctional and powerful devices for health diagnostics, smart prosthetics and rehabilitation, and human-machine interfaces. Additionally, a new class of flexible ECG electrodes based on organic electrochemical transistors (OECTs) is summarized due to their unique properties of signal amplification and high sensitivity.
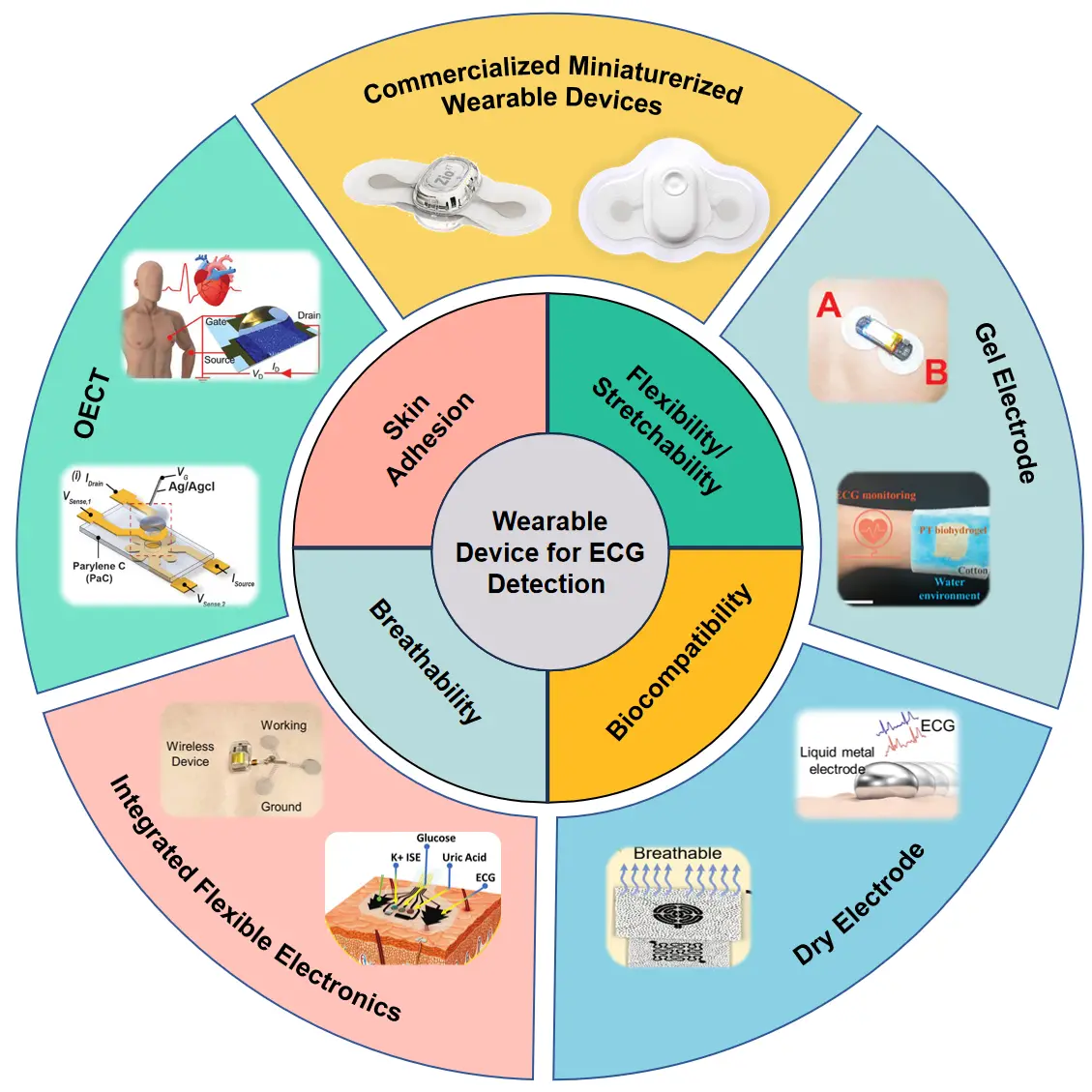
Figure 1. The classification of flexible and stretchable electrode and electronic for wearable ECG detection. Republished with permission from[6,
We review the latest developments specifically related to epidermal electrodes for wearable ECG detection from the perspectives of materials, devices, and skin-integration strategies. This review focuses on the interfacial mechanical and electrical properties of electrodes and electronic devices that enable high signal-to-noise ratio, stability, and user comfort. Although many review papers have addressed flexible wearable electrodes or electronics for biopotential detection, they primarily concentrate on material innovation and the engineering of integrated flexible devices. Few reviews specifically discuss the interfacial mechanical and electrical properties of electrodes or electronic devices essential for high-performance ECG signal recording. The interface between electrodes or electronic devices and the skin significantly affects signal-to-noise ratio, motion artifacts, skin compatibility, and
2. The ECG Signal Acquisition with Epidermal Electrode
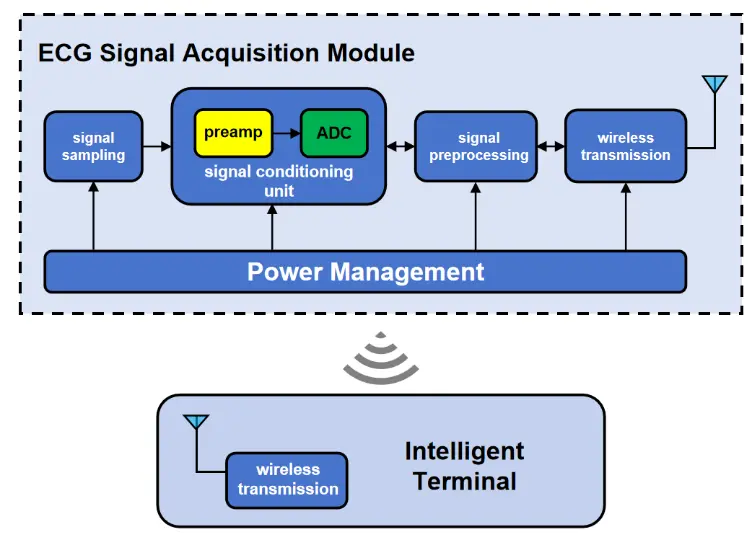
The ECG signal is generated by the weak electrical currents produced during myocardial excitation. These currents propagate from the heart to the limbs and torso, creating measurable potential differences across the body surface. By placing electrodes at specific anatomical locations, these potential differences can be recorded, forming the characteristic waveforms of an ECG[2,16]. In the early 20th century, Willem Einthoven pioneered the first practical electrocardiograph, enabling systematic recording of cardiac electrical activity as waveforms. Decades later, Norman Holter developed the ambulatory ECG monitoring system, revolutionizing continuous cardiac assessment. These innovations laid the foundation for modern electrocardiography, transforming both clinical diagnostics and cardiovascular research[17]. An ECG machine detects the heart’s electrical activity through electrodes placed on the body surface. The raw ECG signals captured by surface electrodes are inherently weak (0.05-5 mV amplitude, 0.05-125 Hz bandwidth) and require sophisticated conditioning. The acquisition module first amplifies these signals and applies bandpass filtering (0.05-150 Hz) to suppress noise from motion artifacts (0.1-10 Hz), powerline interference (50/60 Hz), and baseline drift (< 0.5 Hz). The conditioned analog signals are then digitized by an analog-to-digital converter in the main control unit, achieving a sampling rate of
As skin-contact sensing interfaces, epidermal electrodes play a critical role in acquiring high-quality ECG signals, especially in wearable monitoring applications. These electrodes adhere directly to the skin surface and must maintain stable electrical contact while minimizing motion artifacts and noise interference to ensure reliable signal acquisition during continuous monitoring. The interfacial electromechanical performance of epidermal electrodes, particularly their elastic modulus and interfacial adhesion strength, critically determines their conformability to skin and contact stability. These mechanical properties, together with the electrode-skin interfacial impedance characteristics, directly influence the noise levels in ECG recordings. Therefore, the interfacial electrical and mechanical properties of epidermal electrodes or electronic devices are highly significant for recording high-quality ECG signals. Currently, Ag/AgCl gel electrodes are widely used as cost-effective wet electrodes for ECG signal detection due to their good skin contact and high signal-to-noise ratio. However, a prominent drawback of gel electrodes is their poor long-term stability caused by solvent volatilization in the conductive paste or gel electrolyte. Moreover, the electrolyte in gel electrodes can easily trigger skin irritation. Consequently, traditional gel electrodes are not optimal for long-term wearable ECG monitoring. In contrast, dry electrodes, which provide a stable sensing interface and good biocompatibility, represent an excellent alternative for long-term wearable ECG applications[19,20]. The skin contact behaviors of wet and dry electrodes can be analyzed through interfacial equivalent circuit models (Figure 3). The main difference between the equivalent models lies in the medium layer between the electrode and the epidermis. Wet electrodes rely on conductive gel to maintain good electrical contact with the skin, whereas dry electrodes attach to the skin through an air gap. Specifically, in gel electrodes, the series resistance Rl represents the effective resistance at the gel electrode-skin interface (Figure 3a). The underlying epidermal layer behaves as a parallel circuit of resistance Rs and capacitance Cs. The dermis and subcutaneous layers generally act as pure resistances Rsub. For dry electrodes, the air gap replaces the gel electrolyte function. The electrode-skin interface can be modeled as a contact resistance Rl and contact capacitance Ct in parallel (Figure 3b), resulting in higher contact impedance compared to gel electrodes[21].
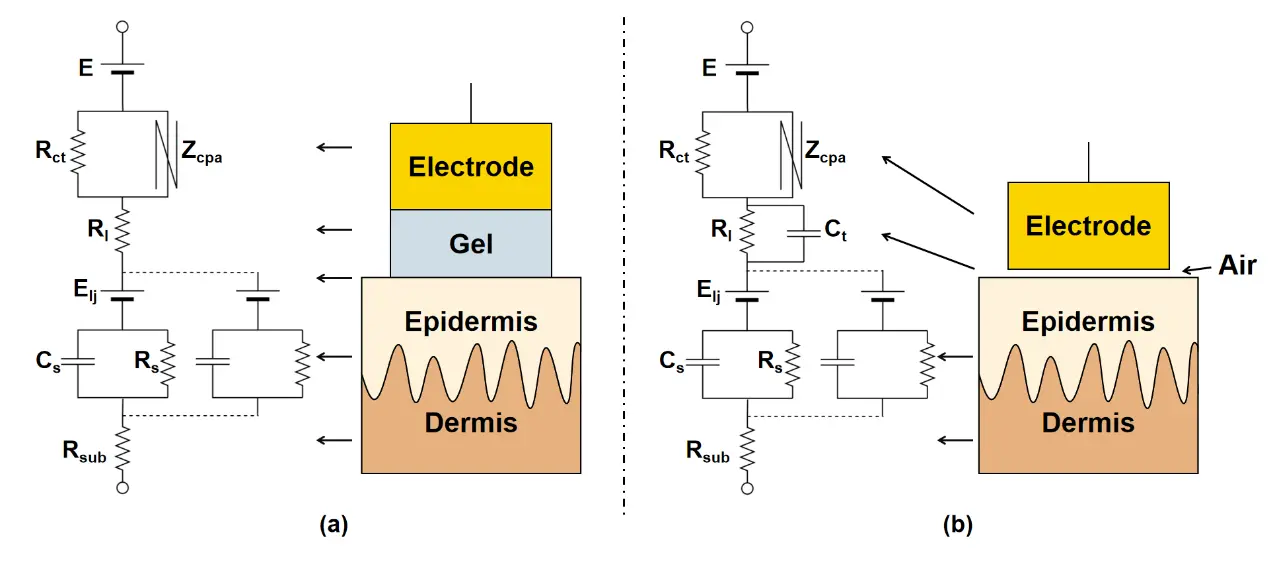
Figure 3. The equivalent circuit of the electrode-to-skin interface for both the gel (a) and dry (b) electrodes.
3. The Requirements for Epidermal Electrodes
The dynamic and multimodal mechanical deformations of human skin, including bending, stretching, compression, and torsion, impose stringent requirements on epidermal bioelectronics. To maintain stable functionality during long-term continuous ECG monitoring, an ideal skin-interfaced electrode must simultaneously provide robust yet comfortable adhesion, tissue-like stretchability (greater than 30% strain tolerance), and mechanical durability. The following sections systematically discuss these critical features for high-performance epidermal electrodes, focusing on key interfacial properties such as flexibility and stretchability design, skin adhesion, breathability, and biocompatibility.
3.1 Flexibility/stretchability
The mechanical flexibility and stretchability of epidermal electrodes are key factors determining user comfort and device portability. These properties enable conformal adhesion to the skin and reliable signal acquisition during dynamic movements, which explains the increasing global research interest in developing advanced skin-conformal electrode materials and architectures. For wearable ECG signal detection, epidermal electrodes or wearable devices are inevitably exposed to various mechanical stresses and deformations. Therefore, it is essential for epidermal electrodes to maintain their functionality under such conditions. Wearable electrodes or devices with good flexibility and stretchability can conform closely to the body. Even on deformable skin during movement, flexible or stretchable electrodes maintain stable contact interfaces, ensuring consistent signal quality. Recent years have witnessed significant advancements in intrinsically stretchable functional materials and sophisticated geometric engineering to achieve flexible and stretchable electrodes.
In addition to flexible and stretchable elastomer substrates, intrinsically stretchable conductive materials include metallic and carbon-based nanomaterials (such as metal nanoparticles, carbon nanotubes, and graphene), conductive polymers, organic semiconducting polymers, and nanocomposites. These conductive components are incorporated into elastomeric polymer substrates, forming deformable and conductive pathways within the composites that exhibit high conductivity and reversible deformation capabilities. Using these materials, intrinsically stretchable skin-like electrodes with a high degree of durability and conformability can be fabricated. Soft conductive electrodes conform well to the skin, increasing the contact area and user comfort. They also improve the electrode’s resistance to motion artifacts. However, it should be noted that these conductive composites often involve a trade-off between mechanical and electrical properties. High loading of conductive fillers can enhance conductivity but may compromise stretchability. To achieve better flexible or stretchable electrodes with good mechanical softness, intrinsically stretchable ionic electrode materials are employed to prepare soft epidermal electrodes. For example, a poly(ionic liquid) gel (PILG) electrode was developed via photoinitiated polymerization of the ionic vinyl monomer 1-vinyl-3-butylimidazolium bis(trifluoromethyl sulfonyl)imide and a cross-linker in an ionic liquid medium. The resulting PILG exhibited exceptional ionic conductivity and good mechanical resilience. Notably, the electrode demonstrated rapid shape recovery after deformation and maintained stable stretchability and conductivity underwater, highlighting its suitability for dynamic environments such as aquatic ECG monitoring[22]. However, the potential skin irritation caused by ionic electrolytes remains a concern, especially for long-term
On the other hand, to achieve stretchability with rigid materials such as metals or brittle silicon, appropriate deformable geometry designs can be employed to create stretchable structures, including net-shaped, island-bridge, serpentine, and out-of-plane buckles. For example, a stretchable metal dry electrode with hexagonal spring-like structures was developed by optimizing the mechanical and electrical performance of the meander geometry. Compared to conventional circular or rectangular designs, the hexagonal multi-track meander electrode architecture increases surface area, reduces interfacial stress concentration, and effectively suppresses electrode buckling and skin detachment during stretching. Experiments also demonstrated lower impedance and a high signal-to-noise ratio (88 dB) comparable to wet electrodes (87 dB), along with superior resistance to motion artifacts[24]. Additionally, second-order serpentine structures offer a new paradigm for highly stretchable electronics. A stretchable carbon nanotube (CNT)-based smart electronic tape (SET) with second-order serpentines was fabricated on biocompatible substrates. Mechanical simulations revealed that, compared to linear or first-order serpentine patterns, the multi-curve geometry homogenizes stress distribution, achieving a resistance change rate below 40% at 100% strain and significantly reducing low-frequency baseline noise during deformation. This deformable structure enables SET to achieve over 300% stretchability while maintaining stable electrophysiological signal acquisition[25].
3.2 Skin adhesion
During body movement or skin deformation, fluctuations in the electrode–skin contact area generate dynamic noise, which negatively affects ECG signal acquisition, recognition, and interpretation. This dynamic noise at the bioelectric electrode-skin interface poses a major challenge for wearable ECG devices. Skin electrodes or electronics ideally possess robust skin adhesion and good mechanical durability to ensure stable functionality during long-term, continuous biopotential monitoring. Sufficiently thin and flexible materials and devices can adhere to human skin through pure van der Waals forces. Such self-adhesive thin electrodes can bond to the skin without the need for adhesive gels or external forces. For example, a thin-film tattoo dry electrode approximately
For long-term wearable ECG monitoring, adhesive epidermal electrodes with good mechanical robustness, are essential due to the constant and dynamic motions of the skin. Recently, advanced materials and innovative structures have been developed to enhance skin adhesiveness without compromising mechanical durability. For example, a self-adhesive stretchable Ag nanoparticles/polyurethane dry electrode featuring biomimetic hook-like structures inspired by burdock fruits has been developed to increase the contact area and interfacial adhesion between the electrode and the skin[27]. Compared to planar electrodes, these adhesive electrodes with microstructures exhibit a higher dynamic friction coefficient, offering excellent resistance to hair interference and motion artifacts during body movement. They can stably record high-quality ECG signals with low noise levels during dynamic activities. Similarly, some dry electrodes with micro- or nano-pillar array structures have been fabricated using templated methods to enhance microscale interfacial interactions between the electrode and skin[28]. These microstructured dry electrodes not only conform well to human skin but also show improved signal quality and stability, which is particularly beneficial for dynamic ECG measurements by reducing motion artifacts. To improve adhesion on hairy skin, viscoelastic soft electrodes have been developed using semi-fluidic or highly stretchable materials that facilitate penetration through hair[29]. By conformally adapting to the microscale topography of hairy skin, these electrodes maintain low interfacial impedance even during dynamic motion, demonstrating applicability for hairy skin and long-term wearable monitoring. This significantly reduces motion artifacts and enables stable wearable ECG monitoring.
3.3 Breathability
While most flexible epidermal bioelectronics use impermeable substrates for device stability, optimal wearable ECG monitoring requires breathable interfaces that maintain skin homeostasis. Such interfaces should balance permeability to air, water vapor, and liquid perspiration while preserving electrical performance, a critical but often underexplored design paradigm for long-term biocompatibility. Breathable electrodes or electronic devices improve skin compatibility by reducing the risk of irritation. Porous textile architectures and nanofiber networks offer exceptional breathability (air permeability > 1000 L/m2/s) and tunable mechanical properties, making them ideal substrates for gas-permeable epidermal electrodes in wearable bioelectronics[30]. Textile-based electrodes have gained prominence due to their structural versatility (knitted, woven, or nonwoven configurations), inherent skin compatibility, and natural moisture management. Through controlled deposition of conductive inks such as
On the other hand, breathable electrodes or electronics can be fabricated using nanofiber networks as porous substrates. Electrospun polymer nanofibers are among the most commonly used materials for breathable electrodes or electronics due to their excellent permeability, tunable configurations, and superior mechanical properties. With appropriate material composition, surface functionalization, and architectural design, polymer composite nanofibers can be employed to fabricate breathable electrodes with excellent skin conformability and adhesiveness[34]. For example, Guo et al.[35] proposed a sandwich-structured breathable electrode using polypropylene electrospun nanofiber films as the substrate and semi-liquid metal as the conductive component. This breathable and stretchable nanofiber film electrode conforms well to the skin and provides good comfort, enabling continuous 24 hours ECG signal measurement. The ECG signals recorded by these breathable electrodes exhibit higher quality than those from commercial Ag/AgCl electrodes. To achieve breathable electrodes with continuous perspiration capability, a Janus textile electrode with differing surface wettability was fabricated using superhydrophilic hydrolyzed polyacrylonitrile nanofibers and superhydrophobic polyurethane/Ag nanowires composite nanofibers. This all-nanofiber-based Janus epidermal electrode features two opposing wettability surfaces, an asymmetric hydrophilic upper layer and a hydrophobic bottom layer, which allows the electrode film to effectively transport sweat to the upper surface while maintaining good breathability. The daily water loss of the Janus textile electrode is significantly higher than that of commercial gel electrodes, and its water vapor transmission rate (WVTR) reaches 1748.09 g·m-2·d-1, far exceeding both the WVTR of human skin and values reported in existing literature. This demonstrates superior breathability without causing skin irritation or allergic reactions during long-term wear[36]. Eskandarian et al.[37] used a 3D knitting machine to weave conductive elastic fibers (CEF) into dry textile electrodes, seamlessly integrating them into garments for long-term, comfortable ECG monitoring. The optimized CEF electrode exhibits a WVTR of 20.05 g·m-2·h-1, surpassing the natural WVTR of skin and ensuring high breathability. Prototypes of smart underwear and headbands demonstrated stable ECG signal recording with an average SNR of 18.32, indicating both good wearing comfort and signal accuracy.
3.4 Biocompatibility
Biocompatibility is a crucial design criterion for wearable ECG electrodes and devices due to their prolonged contact with the skin, especially in long-term monitoring applications. Significant efforts have been dedicated to the development of biocompatible conductive materials, stretchable substrates, and encapsulation layers[38]. To minimize skin irritation, biodegradable materials or natural polymers are commonly employed in electrode fabrication[39]. For instance, Hu et al.[40] developed a biocompatible electrode patch using ductile poly(methyl vinyl ether-alt-maleic acid) (PMVEMA), a water-soluble polymer with excellent biocompatibility and adhesive properties. This allows the electrode to adhere to various surfaces autonomously and significantly improves the interfacial stability and quality of signal acquisition. In addition, the incorporation of biocompatible glycerin acts as a plasticizer, enhancing flexibility and reducing solvent evaporation, thereby maintaining stable electrical performance. This formulation effectively addresses both mechanical adaptability and long-term operational stability while maintaining skin compatibility. The resulting electrodes are capable of recording high-quality ECG signals with a SNR exceeding 20 dB, clearly distinguishing the P wave, QRS complex, and T wave to ensure accurate signal capture. Natural materials such as potato peel and silk protein have also been explored as biocompatible substrates for skin-contact electrodes. Potato peel is non-irritating and possesses antibacterial and antioxidant properties. Its moist inner surface can be directly applied to the skin to create innovative natural electrodes, offering performance comparable to that of conventional Ag/AgCl electrodes.[41]. Natural silk fibroin (SF), a natural protein, has emerged as a promising material for hydrogel-based epidermal electrodes due to its excellent conformability and biocompatibility. When electrospun into nanofibers, SF forms a three-dimensional microporous network that supports breathability, moisture management, and close skin adhesion. For instance, a soft, wet-adhesive SF nanofiber film electrode was prepared using a three-layer structure composed of polytetrafluoroethylene nanofibers, silver nanowires, and SF nanofibers. These fabric electrodes demonstrated high conductivity (approximately 3.58 Ω/sq), excellent breathability (51.5 mm/s), and a water vapor transmission rate of 2,553 grams per square meter per day at 35 °C, along with good adhesion to moist skin. The electrodes maintained firm contact without causing noticeable irritation and provided a stable skin-electrode interface, enabling continuous ECG monitoring with high signal quality (SNR around
4. Flexible/Stretchable Epidermal Electrodes for Wearable ECG Signal Detection
ECG monitoring, as a fundamental technology for diagnosing cardiovascular diseases and enabling real-time health surveillance, is highly dependent on electrode performance to ensure high-quality signal acquisition. The development of wearable ECG monitoring has shown a clear evolution from wet electrodes to dry electrodes and eventually to fully integrated devices (Figure 4). Wet electrodes, initially adopted as the clinical standard, rely on conductive gel to minimize interfacial impedance. However, they present several limitations, including gel dehydration during prolonged use and the risk of skin irritation or allergic reactions[46]. To address these issues, next-generation dry electrodes have been designed using advanced material structures and surface engineering approaches that eliminate the need for hydrogels, thereby supporting long-term usability[27,28]. Modern dry electrodes now exhibit a wide range of architectures. They have evolved from early flexible metallic films to more sophisticated stretchable designs incorporating serpentine microstructures. These metallic dry electrodes show greatly improved conformability and stability at the skin interface. Their structural reliability also allows for direct integration with electronic components, enabling the development of multifunctional wearable systems capable of simultaneous biosignal acquisition, signal processing, and wireless transmission. Among recent advancements, the most cutting-edge dry electrodes are fabricated from intrinsically stretchable conductive polymers with mechanical properties closely matched to human skin. These next-generation electrodes combine key attributes such as tissue-like compliance, optimized interfacial adhesion, and intrinsic noise reduction, which together facilitate reliable, long-term ECG monitoring with minimal interference from motion artifacts. Beyond material-level improvements, current research efforts are increasingly focused on system-level integration. The goal is to develop lightweight, stretchable wearable systems with multimodal sensing capabilities, including ECG, temperature, biochemical signals, and modules for biomedical diagnosis or treatment. Enabled by advanced fabrication techniques, these integrated platforms support comprehensive health monitoring through synchronized,
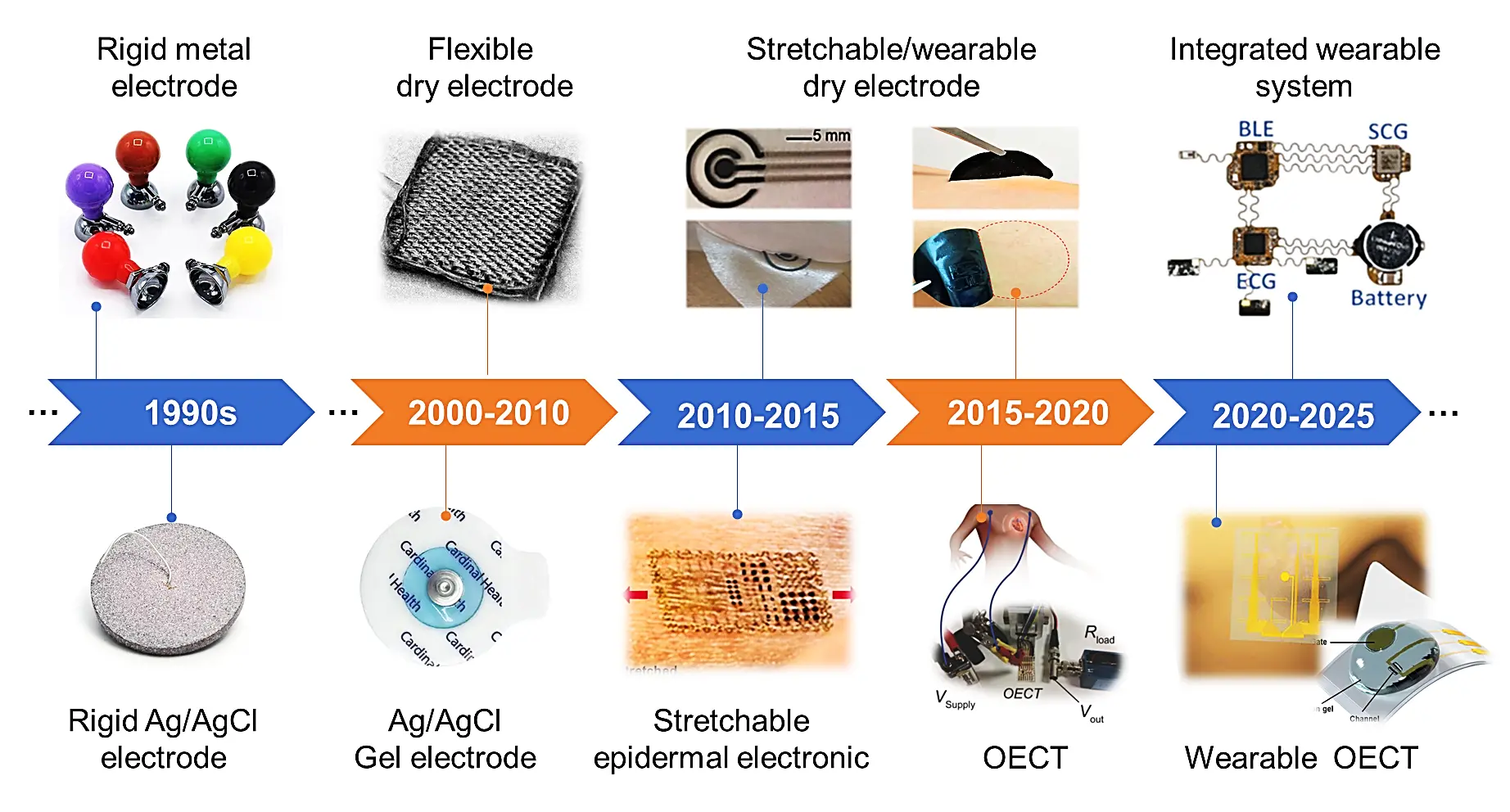
Figure 4. The development timeline of wearable epidermal electrodes and devices for wearable ECG monitoring from 2000 to 2025 year. Republished with permission
Recently, an emerging type of ECG sensor based on OECTs has been developed, showing strong potential for use in lightweight,
4.1 Gel electrode
Conventional wet electrodes, exemplified by Ag/AgCl hydrogel electrodes, offer excellent signal-to-noise ratio and signal stability during short-term monitoring by converting ionic signals to electronic signals through conductive hydrogels. Currently, these gel electrodes remain the clinical standard. However, their inherent limitations restrict their suitability for prolonged continuous monitoring. Water evaporation leads to hydrogel dehydration, which significantly reduces conductivity and results in signal attenuation or even electrode failure[49]. In addition, the electrolytes in ionic conductive hydrogels can cause skin irritation and, in some cases, allergic contact dermatitis. Recent research aims to address these challenges through innovations in materials and structural design. The goal is to develop next-generation wet electrodes that combine enhanced biocompatibility, mechanical compliance, and sustained hydration. These advancements seek to ensure high-fidelity ECG signal acquisition while creating flexible, skin-conforming interfaces that better accommodate the dynamic nature of human skin.
To address concerns about the limited interfacial biocompatibility of synthetic polymer hydrogels, natural biomaterials such as silk fibroin and gelatin have emerged as promising alternatives for biocompatible hydrogel electrodes[50]. Silk fibroin, a natural biopolymer derived from silk, offers excellent biocompatibility, unique mechanical properties, tunable degradation rates, and easy solution processability. Conductive nanomaterials or polymers can be incorporated into silk fibroin to fabricate skin-friendly electrodes. Recently, a silk fibroin-based double-network hydrogel (PAM-SF/Fe3+) with ionic conductivity was fabricated using a metal chelation and hydrogen-bonding self-assembly strategy, achieving excellent biocompatibility and wet-tissue adhesion. This hydrogel demonstrated cell proliferation comparable to blank controls, confirming its low toxicity and biosafety. As a stretchable and adhesive interfacial electrode, it adhered stably to the skin under sweat conditions and delivered ECG signals with clear PQRST waveforms equivalent to commercial Ag/AgCl electrodes. However, this ionic conductive hydrogel exhibits low conductivity (0.2 S/m). ECG recordings from this hydrogel electrode showed a low signal-to-noise ratio and moderate baseline drift over short durations[51]. As a highly conductive nanofiller, MXene can be combined with silk fibroin to produce biocompatible hydrogel electrodes. You et al.[52] developed a conductive hydrogel (PSDM) by incorporating MXene and dopamine-modified polypyrrole (PPy) as conductive enhancers within a silk fibroin/polyvinyl alcohol (PVA) gel network. The composite hydrogel exhibited remarkable electrical conductivity
In wet gel electrodes, ionic electrolytes act as the primary conductive medium due to their aqueous properties and
Some ions at high concentrations may disrupt the skin barrier function through osmotic imbalance or direct chemical interactions, potentially leading to skin irritation, compromised cell membrane stability, and inflammatory responses. To improve the biocompatible and comfort of gel electrodes for long-term use, highly conductive polymers or carbon nanostructures are selected as alternative conductive components in hydrogel electrode to balanceconductivity and biosafety synergistically. Yao et al.[57] developed a conductive nanocomposite hydrogel electrode (FPCH) composed of poly(ether) F127 diacrylate (F127DA), single-walled carbon nanotubes (SWCNTs), and PEDOT:PSS via 3D printing. Benefiting from the high conductivity of PEDOT:PSS and SWCNT, this hydrogel electrode exhibits exceptional stretchability (up to 520% strain), high conductivity (~440 S/m), and excellent biocompatibility. As an epidermal ECG electrode, the FPCH electrode captured ECG signals with peak-to-peak amplitudes three times higher than commercial gel electrodes due to its low interfacial impedance (~100 Ω at 10 Hz) and conformal skin contact, yielding sharp PQRST waveforms and an enhanced SNR (18.3 dB) (Table 1).To further enhance conductivity, another study incorporated PEDOT:PSS and graphene into a hydrogel with a dual crosslinked network of gelatin and PVA, forming the GPH hydrogel. The improved conductivity results from the synergy between the conjugated structure of PEDOT:PSS, which facilitates electron mobility, and the conductive network formed by graphene sheets, enabling efficient electron and ion transfer. The conductivity remains stable at approximately 27.3 S/cm over 14 days. For ECG monitoring, the PEDOT: PSS/graphene/GPH hydrogel electrode delivers performance comparable to intact electrodes. The high SNR (34.9 dB) confirms its reliable stability for high-fidelity ECG acquisition[58]. Organizing conductive components into a connected network can impart high electrical conductivity to composites. To improve conductivity, He et al.[59] fabricated a conductive aramid nanofiber film electrode via in-situ polymerization of PPy on aramid nanofiber. The conductive PPy layer on the hyperconnected nanofibrous networks established a continuous conductive pathway, achieving ultrahigh conductivity (~106 S/cm). Cardiomyocyte cultivation experiments demonstrated high viability with significant expression of troponin T and connexin-43, verifying excellent biocompatibility. With low interfacial impedance (30 Ω cm2 at 10 Hz) and high charge injection capacity, the electrode captured high-quality ECG signals with a NR greater than 32 dB (Table 1), displaying distinct QRS complexes,
| Electrode types | Conductive Component | Interfacial Impedance | Flexibility/Stretchability | SNR | Ref. |
| Wet electrode | Salt ions | ~80 Ω/100 Hz | > 400% | 37.8 dB | [6] |
| Ionic liquids | N.R. | 1,476 ± 24% | N.R. | [56] | |
| CNT/PEDOT:PSS | ~100 Ω/10 Hz | 520% | 18.3 dB | [57] | |
| PEDOT:PSS | N.R. | ~400% | 34.9 dB | [58] | |
| PPy nanofiber | ~30 Ω/10 Hz | > 80% | N.R. | [59] | |
| Liquid metal | 70 kΩ/100 Hz | 550% | ~35 dB | [60] | |
| Dry electrode | Metal | < 3 kΩ/1 kHz | > 350% | 20.4 dB | [61] |
| MXene | 4.68 kΩ/1 kHz | > 40% | 16.5 dB | [62] | |
| PEDOT:PSS | 48.2 kΩ/100 Hz | 62% | N.R. | [63] | |
| OECT | PEDOT:PSS | N.R. | Flexible | 42.5 dB | [2] |
| PEDOT:PSS | N.R. | Flexible | 20.6 dB | [64] | |
| Integrated electronics | CNT@NPC | ~1 MΩ | 350% | 26 dB | [15] |
| Metal/ionic gel | N.R. | Flexible | 35 dB | [65] | |
| Metal/ionic liquid | N.R. | > 20% | N.R. | [66] |
SNR: signal-to-noise ratio; ECG: electrocardiograph; CNT: carbon nanotube; NPC: nanoporous carbon; OECT: organic electrochemical transistors;
For wearable ECG monitoring, the interfacial adhesion of gel electrodes on the skin is crucial for maintaining stable skin contact, which directly influences signal quality by reducing motion artifacts and interfacial impedance. Gel electrodes with tunable adhesion properties minimize noise during movement and ensure prolonged wearability, even in humid or underwater environments[67]. Inspired by the natural moisturizing factor in the stratum corneum, a thermos-responsive adhesive hydrogel based on
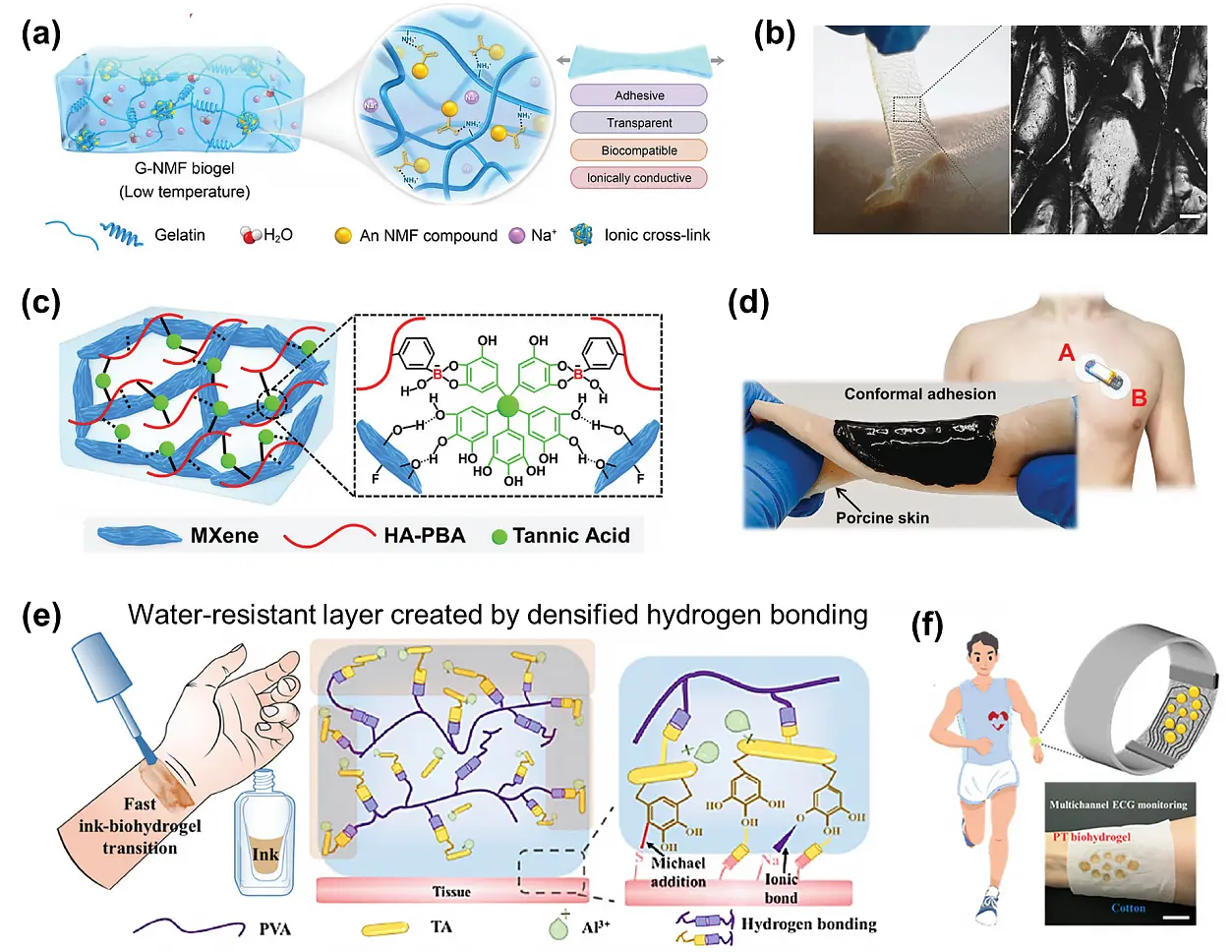
Figure 5. Wearable wet epidermal electrodes. (a), (b) Adhesive ionic hydrogel electrode and its good conformability with skin. Republished with permission from[68]; (c), (d) Preparation of an adhesive, healable, and antibacterial MXene/HA-PBA/TA hydrogel electrode for wireless wearable ECG measurement. Republished with permission from[10]; (e) A paintable waterproof PVA-TA (PT) hydrogel based on dense hydrogen-bonded networks. Republished with permission from[6]; (f) A wearable ECG recording electrode by printing PT hydrogel on a cotton cloth. Republished with permission from[6]. HA-PBA: phenylboronic acid grafted hyaluronic acid; ECG: electrocardiograph; TA: tannic acid; PVA: polyvinyl alcohol.
Human skin exhibits microstructured wrinkles and a hairy surface, posing challenges for conventional hydrogel electrode patches to achieve conformal contact, especially at the microscale. To ensure tight adhesion on such microstructural skin, precise modulation of the hydrogel electrode’s mechanical modulus is essential. A conformally self-adhesive epidermal hydrogel electrode was developed by combining conductive MXene nanosheets with a supramolecular hydrogel composed of natural polyphenol tannic acid (TA) and phenylboronic acid-grafted hyaluronic acid (HA-PBA). Within the hydrogel system, dynamic borate ester bonds and supramolecular interactions between HA-PBA and TA synergistically reduce the mechanical modulus to a lower range. This reduction enables conformal attachment to porcine skin without visible interfacial gaps. Moreover, the abundant catechol groups in TA provide strong, repeatable adhesion to various substrates. By leveraging the high-adhesive interface and high conductivity, the hydrogel achieves low-impedance, conformal skin contact, significantly enhancing the SNR of ECG signals. The sensor stably monitors ECG signals for up to 12 hours, clearly capturing P-waves, QRS complexes, and T-waves, offering reliable data for diagnosing arrhythmias and related disorders (Figure 5c and Figure 5d)[10].
Under real-world conditions, perspiration or water exposure moistens the skin surfaces, significantly compromising the adhesion of hydrogel electrodes. This moisture-induced interfacial weakening substantially degrades electrode performance in wet environments due to three primary mechanisms: (1) formation of an interfacial hydration layer that disrupts molecular adhesion, (2) potential leakage of conductive components, and (3) swelling-induced structural changes caused by interactions among hydrophilic groups. Consequently, developing hydrogel electrodes with robust adhesion in aquatic environments has become a critical focus in flexible bioelectronics research[69].
To enhance the environmental adaptability of electrodes, current research primarily focuses on developing materials with waterproof properties to ensure stable operation under diverse conditions. For example, Luo et al.[6] fabricated a waterproof ionic conductive hydrogel by creating dense hydrogen-bonded networks formed between TA and PVA. In this system, water-induced densification of hydrogen bonds strengthens the network’s compactness, effectively blocking water penetration while preserving structural integrity. The hydrogel was integrated onto cotton fabric to produce electrodes for ECG monitoring. The resulting electrodes exhibit low skin-contact impedance (80 Ω at 100 Hz) and excellent stretchability (Table 1), enabling stable and high-quality ECG signal acquisition even in perspiration or aquatic environments. Notably, distinct ECG waveforms remained clearly detectable after 72 hours of continuous immersion in simulated sweat, with no significant increase in noise (Figure 5e and Figure 5f).
4.2 Dry electrode
Despite significant research progress, gel-based electrodes remain inherently limited by their reliance on solvents. These systems encounter two major challenges: gradual performance degradation due to solvent evaporation, and the risk of inflammatory reactions on sensitive skin caused by migrating ionic components[55,70]. Dry electrodes address these issues by adopting solvent-free designs that integrate novel materials with advanced microfabrication techniques. This strategy achieves interfacial impedance comparable to that of wet electrodes while ensuring wearer comfort and biocompatibility[71]. Dry electrodes provide three main advantages for long-term health monitoring: (1) enhanced skin conformability, (2) reduced skin irritation, and (3) superior biocompatibility[27,28]. Nonetheless, their application in ECG monitoring still faces a fundamental obstacle, poor initial skin contact, which leads to increased interfacial impedance and greater susceptibility to motion artifacts[49].
To address these challenges, advancements in material innovation and structural engineering have accelerated the development of wearable dry electrodes. The current material portfolio for dry electrodes mainly comprises three categories: metal-based
As a representative example of dry electrodes, metal electrodes constitute the most established class, having been widely adopted in commercial wearable devices and medical instruments due to their exceptional mechanical durability and superior electrical conductivity. In recent years, various smart wearables with ECG detection functions have attracted significant attention across different application scenarios, including medical health monitoring and consumer-grade use (Figure 6a)[76]. These commercial wearable ECG devices employ metal electrodes to detect epidermal ECG signals. Combined with digital and electronic technologies, smart wearable ECG equipment has enhanced work efficiency and improved the accuracy of clinical diagnosis[77,78]. Wearable patches typically consist of miniature sensor modules containing electronic components and sensing elements, which can be directly affixed to the chest via adhesive surfaces. Their advantages include low-cost manufacturing, feasibility for mass production, superior flexibility, and strong resistance to motion artifacts during physical activity and skin deformation[26]. Furthermore, these patches show potential for functional expansion through integration of additional physiological sensors to monitor diverse parameters such as blood glucose, pH, and body temperature[79,80].
Figure 6. ECG monitoring by the metal electrodes. (a) The rigid metal electrode on a smart watch; (b) Stretchable metal electrode or connection with deformable architecture for conformable skin attachment[43]; (c) Stretchable metal electrode patch with deformable serpentine structures for wearable ECG monitoring[66].
The inherently rigid and sliding nature of metal electrodes often leads to motion artifacts caused by electrode displacement on the skin, resulting in severe noise interference. To improve the performance of metal electrodes and specifically address motion artifacts, flexible and stretchable metal electrodes with innovative structural designs have been developed in recent years. John A. Rogers’s group developed stretchable thin-film metal electrodes featuring carefully designed deformable geometries, such as serpentine patterns (Figure 6b). These metal electrodes demonstrate skin-like softness and good stretchability through geometric engineering, significantly enhancing skin conformability and reducing motion artifacts caused by displacement. Such deformable structural designs, which incorporate rigid yet durable materials, have been widely adopted for fabricating stretchable dry electrodes[81].
For example, to achieve stretchable and skin-compatible electronics for neonatal monitoring, ultrathin, soft, skin-like electronic devices were designed with serpentine metal traces and an island-bridge connection structure to minimize mechanical stress during deformation[66]. This structure allowed uniaxial stretching up to 16% without plastic deformation. A microfluidic chamber filled with ionic liquid further decoupled strain, reducing interfacial shear and normal stresses by 2.5 times compared to non-fluidic designs. For continuous wearable ECG testing, the epidermal device mounted on the chest captured electrocardiograms via fractal mesh electrodes. Real-time in-sensor analytics processed QRS complexes, providing heart rate, heart rate variability, and respiration rate with clinical-grade accuracy (Figure 6c). Similarly, a stretchable electronic skin made of metal thin-film electrodes was fabricated on a flexible polyimide film[82]. With sophisticated curved geometries and ultrathin designs, these metal electrodes achieve excellent stretchability and interfacial adhesion, enabling seamless conformability to the skin even under dynamic deformation. As a result, electrode-skin impedance was clearly reduced by over 50%, achieving ECG signal stability comparable to that of gel electrodes.
Additionally, to reduce the skin-contact impedance of metal dry electrodes, some metal alloy electrodes have been investigated. By increasing the work function of the alloy composition through metal element doping, the interfacial charge transfer resistance can be lowered by forming a nanoscale electrical double layer at the metal surface, thereby reducing the overall electrode-electrolyte interface impedance of the alloy electrode[83]. For example, Yang et al.[84] designed a Fe-Co-Ni-Cu-Zn high-entropy alloy (HEA) electrode via electrochemical deposition. Its low contact impedance (6.6 KΩ at 1 kHz, superior to conventional dry electrodes) enhances ECG signal fidelity, capturing distinct PQRST waveforms. The HEA electrode demonstrates excellent stability and biocompatibility. The alloy’s optimized charge transport at the skin-electrode interface addresses signal attenuation commonly observed in dry electrodes, proving its feasibility for wearable ECG monitoring. To reduce the interfacial impedance caused by the stratum corneum, Gwak et al.[85] developed a pyramidal microneedle dry electrode using a bismuth-indium-tin (Bi-In-Sn) alloy for high-fidelity ECG monitoring. The microneedles, measuring 340 μm wide and 800 μm high, penetrate only the stratum corneum, reducing impedance to below 10 kΩ (at 10 Hz). ECG signals captured by these electrodes exhibit clear P-wave, QRS complex, and
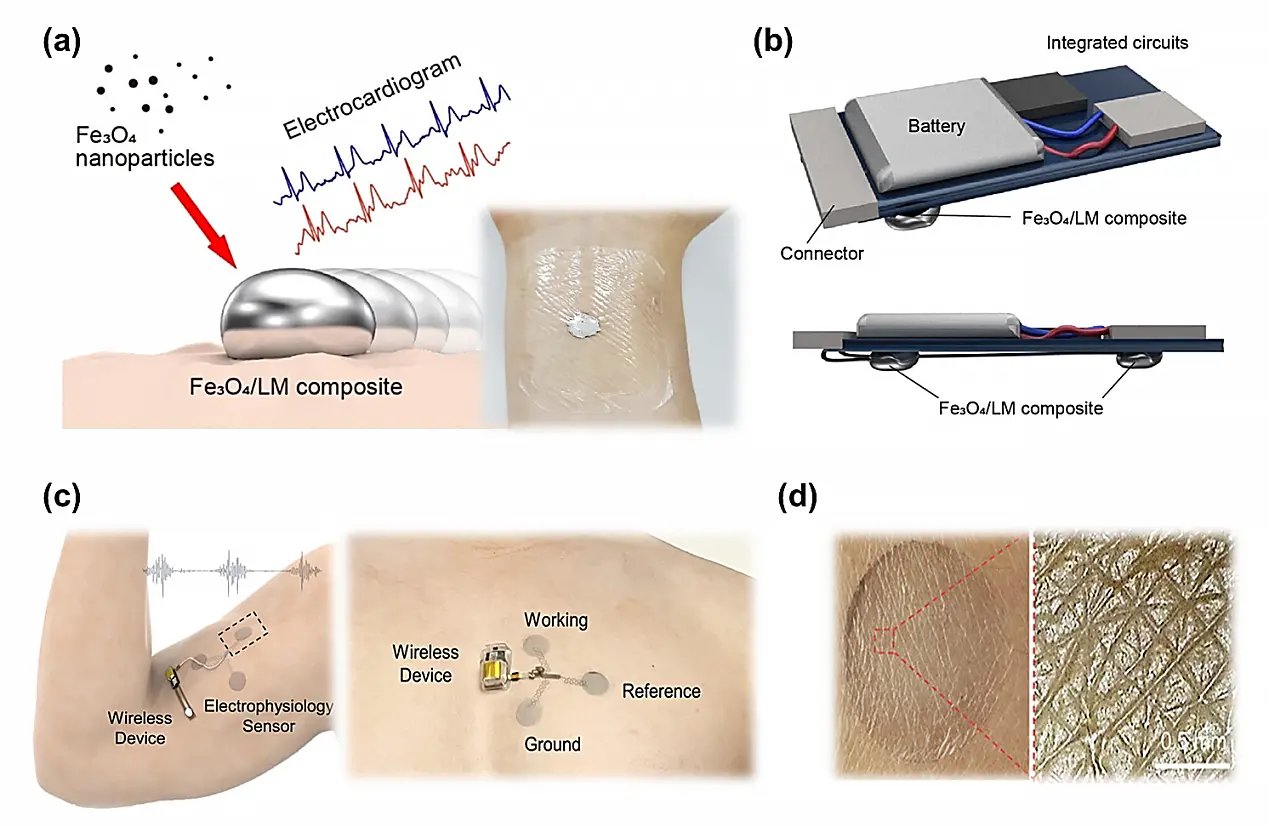
Figure 7. Wearable dry electrode. (a) A highly conductive Fe3O4/ LM composite dry electrode and wireless ECG monitoring system composed of two liquid electrodes (b)[11]; (c) A wireless electrophysiology measurement system composed of conventional ultrathin PPd tattoo electrodes attached to an upper arm or chest. Republished with permission from[12]; (d) Macroscale (left) and microscale (right) conformability of PPd electrodes on the skin. Republished with permission from[12]. LM: liquid metal;
Unlike inherently rigid metal electrodes, dry electrodes based on conductive polymer composites have emerged as ideal flexible substrate materials due to their skin-like modulus, excellent stretchability, and tunable interfacial properties. By incorporating conductive fillers such as carbon nanomaterials into elastomeric polymer substrates, the resulting composites achieve both high conductivity and stretchability[88]. Metal nanowires (NWs), especially silver nanowires (AgNWs), have become prominent conductive fillers in polymer composites owing to their outstanding electrical properties. When integrated into polymer matrices, these nanostructures form low-resistance percolation networks that simultaneously enhance mechanical robustness and electrical conductivity of the composite. This unique combination makes them particularly suitable for flexible electrodes and wearable electronic applications[89]. Harimurti et al.[90] developed a Janus electrode with asymmetric wettability by depositing a microporous Au nanoparticles/polyurethane-polydimethylsiloxane (Au/PU-PDMS) composite membrane on a hydrophilic PVA/polyacrylic acid (PAA) nanofiber layer. The hydrophobic and microporous Au/PU-PDMS layer provides self-adhesion and mechanical flexibility, enabling unidirectional sweat expulsion when combined with the asymmetric hydrophilic PVA-PAA layer. These multifunctional features allow the electrode to maintain a dry and stable skin-electrode interface as well as good gas permeability and comfort. Experimental results showed the electrode exhibited a minimal relative resistance change of approximately 1% after 1,100 mechanical bending cycles, skin impedance comparable to commercial gel electrodes, and stable ECG monitoring for six days with a consistent of about 18.8 dB. Park et al.[91] created a self-healing patch that integrates ECG signal detection with monitoring of other health conditions such as temperature and wrist pulse. Conductive Au nanosheets were capped with a room-temperature self-healing polymer (TUEG3) and subsequently deposited on a self-healing oxime-carbamate (OC) bond-based polyurethane film. The electrode layers self-bond through the self-healing OC bond-based polyurethane, enabling autonomous damage repair during prolonged use and stabilizing the conductive interface. ECG signals recorded by the composite electrode show distinct QRS peak features with a high SNR of approximately 20 dB. Even after self-healing, the electrode retains good performance with only a slight SNR reduction of about 3%, demonstrating strong potential for long-term ECG monitoring applications.
Although conductive polymer composite electrodes exhibit high conductivity and good stretchability, the conductive nanofiller network is sensitive to strain. Such electrodes are easily affected by skin deformation or body movement, which can cause baseline shifts. Therefore, a motion- or strain-insensitive stretchable electrode is highly desirable Kim et al.[61] developed a motion-insensitive dry electrode by employing a structural design based on kirigami-patterned cracks. In this electrode, silver nanowires (Ag NWs) and gold films were successively deposited on a stretchable poly(styrene-ethylene-butylene-styrene) (SEBS) substrate, forming
Carbon nanomaterials including CNTs, carbon black (CB), graphene, and carbon nanofibers represent another important class of conductive fillers for developing stretchable epidermal electrodes with polymer matrices[92]. Their exceptional electrical stability, high conductivity, and cost-effectiveness make them promising candidates for next-generation practical dry electrodes[93].
Intrinsically conductive polymers (ICPs) have emerged as promising materials in bioelectronics. Unlike inorganic conductors, ICPs offer advantages such as high mechanical flexibility, good electronic and ionic conductivity, and excellent biocompatibility. Therefore, they can address issues related to mechanical mismatch and high interfacial impedance in bioelectrodes and devices[96]. PEDOT:PSS, a widely used commercial conductive polymer, has become a material of choice for functional epidermal electrodes due to its excellent aqueous processability, high electrical conductivity (up to 1,000 siemens per centimeter), inherent biocompatibility, and mechanical flexibility. However, pristine PEDOT:PSS electrodes suffer from low intrinsic conductivity, limited stretchability, and poor stability in humid environments, which compromise interfacial stability and signal quality for wearable ECG recording[97]. To overcome these limitations, researchers have explored improvement strategies including doping with additives, post-processing treatments, and material hybridization. For example, Shin et al.[12] developed a conductive adhesive electrode (PPD) by blending PEDOT:PSS with PVA and D-sorbitol. Optimizing the ratio of PVA and D-sorbitol adjusts the material’s modulus and adhesion to enhance electrode-skin conformability. The resulting PPD electrode demonstrated stable electrical performance and low contact impedance, maintaining consistent signal amplitude during seven days (168 hours) of continuous ECG monitoring. Even under motion conditions, it effectively identified characteristic ECG peaks with minimal artifacts and maintained appropriate signal-to-noise ratio levels (Figure 7c and Figure 7d). Similarly, freestanding flexible dry electrodes (PM electrodes) were fabricated by blending PEDOT:PSS with maltitol. The conductivity of PEDOT:PSS was enhanced through ethylene glycol treatment, while simultaneous incorporation of maltitol improved interfacial adhesion (0.46 newtons per centimeter), reduced Young’s modulus to 7 megapascals, and increased stretchability to 62 percent through hydrogen-bond-mediated interactions. These dry electrodes demonstrate excellent skin conformability and low interfacial impedance (48.2 kΩ·cm2 at 100 Hz), outperforming conventional Ag/AgCl gel electrodes in ECG monitoring and other biosignal acquisition applications (Table 1). This performance advantage is attributed to their consistently superior signal quality across various operating conditions[63].
4.3 Organic electrochemical transistor based epidermal electrode
OECTs consist of source and drain electrodes, a gate electrode, and an organic semiconductor channel. Their operation relies on gate-controlled ion injection from an electrolyte to modulate the bulk conductivity of the channel. This ionic-electronic coupling throughout the channel volume enables exceptionally high transconductance. Functioning as amplifiers, OECTs exhibit gate voltage (input) controlled drain current (output) with signal power amplification[98]. These characteristics make OECT-based devices ideal for high-sensitivity biosensing applications. Currently, OECTs show significant potential in wearable ECG monitoring systems due to their unique combination of intrinsic signal amplification, low power consumption (< 1 μW), and mechanical flexibility[99,100]. For ECG signal detection, the gate electrode of OECT devices generally interfaces with the skin. Epidermal biopotentials are transmitted to the channel via electrolyte layers and modulate the doping state of the semiconductive polymer in the channel, thereby converting epidermal biopotential signals into electronic signals. The micro-sized OECT can be fabricated on ultra-thin or stretchable substrates, ensuring conformal skin contact, reducing motion artifacts, and enhancing SNR[48].
Although OECTs were initially developed for ECG recording, their conventional aqueous electrolyte systems are not compatible with wearable applications. Currently, reports on miniaturized wearable OECT-based ECG sensors remain limited. To address this challenge, Yang et al.[2] developed a flexible OECT sensor array incorporating an ionic gel electrolyte layer (Figure 8a and Figure 8b). Fabricated on an ultrathin parylene substrate, the device features ionic gel-coated channel and gate regions to ensure optimal skin contact. This unique design demonstrates remarkable mechanical robustness, maintaining stable performance through more than 500 bending cycles. The OECT array leverages its intrinsic signal amplification and narrow channel architecture (10 μm width) to achieve high-fidelity ECG monitoring, exhibiting a signal-to-noise ratio exceeding 40 dB with sub- μs response times (Table 1). These characteristics position this integrated flexible platform as a promising solution for next generation wearable and portable ECG monitoring systems. To address the challenges of signal amplification ability and mechanical stability in conventional OECTs, a
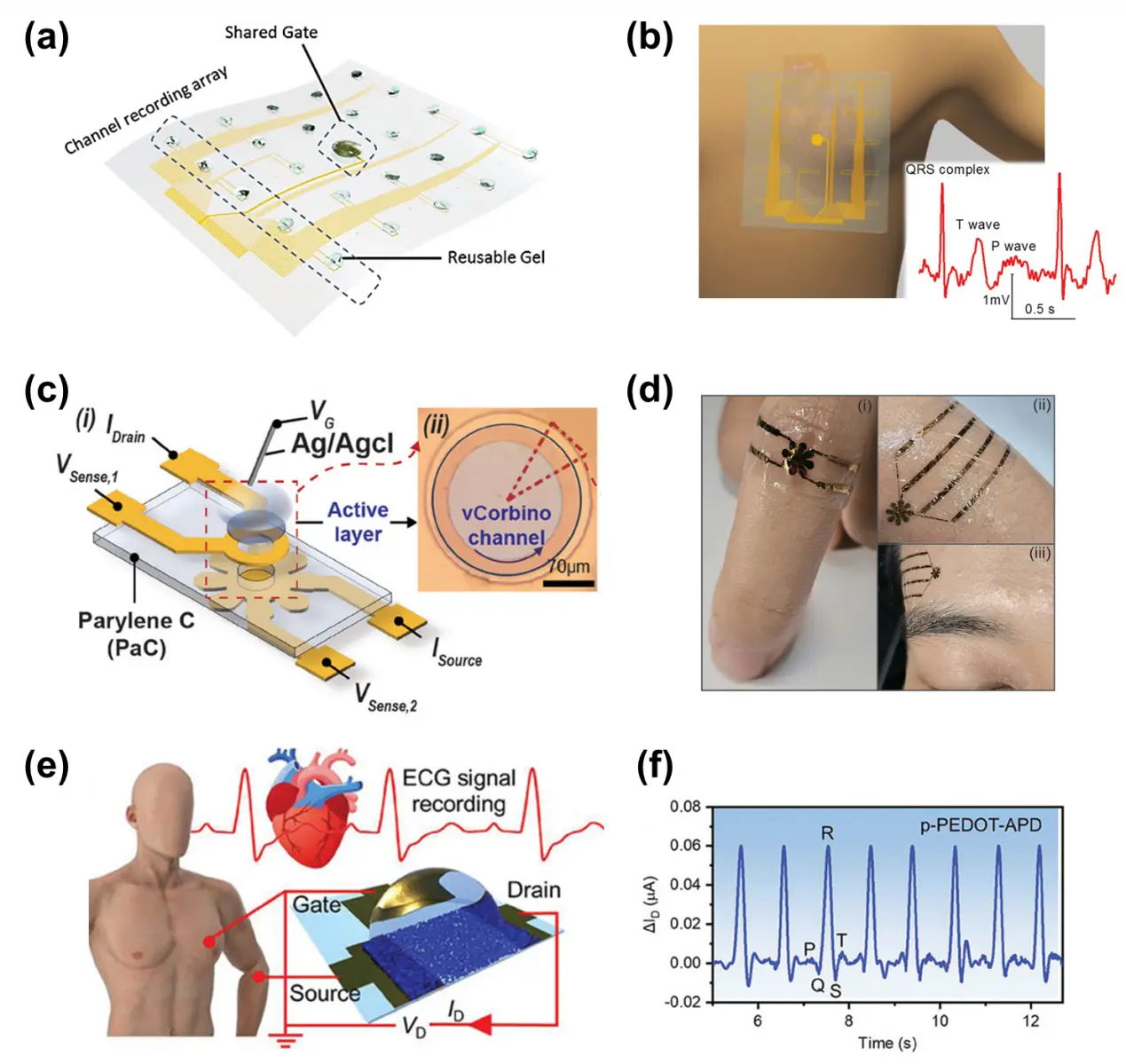
Figure 8. Development of OECT. (a) Illustration of flexible OECT array equipped with 24 channels and 1 shared gate electrode covered with reusable gel[2]; (b) OECT array conformably adhere on a human body for ECG mapping[2]; (c) A 4-T vcOECT device configuration with a vertical Corbino channel region. Republished with permission from[14]; (d) Skin-conformable 4-T vcOECT on the body. Republished with permission from[14]; (e), (f) ECG recording by a OECT device with aerogel-based channel. Republished with permission from[13]. OECT: organic electrochemical transistor; ECG: electrocardiograph; APD: ambient pressure drying; p-PEDOT-APD: PEDOT-based aerogel films prepared by ambient pressure drying method.
To improve the flexibility and mechanical stability of OECTs Jiang et al.[101] constructed a high-performance flexible OECT device using 3D-printed graphene oxide/carbon nanotubes as stable gate, source, and drain electrodes. These 3D-printed electrodes exhibit outstanding mechanical flexibility and porosity, enabling high-throughput fabrication and planar integration while facilitating rapid ion and charge transport. Specifically, the OECTs achieved performance comparable to Ag/AgCl-gated counterparts, with a low threshold voltage and normalized transconductance exceeding 75 S/cm2. At a gate bias of -0.1 V, the recorded current signals displayed frequency and waveform characteristics highly consistent with typical ECG patterns, validating the feasibility of this bioelectronic approach. The volumetric ionic-electronic coupling mechanism in OECT channels fundamentally limits device response time, which in turn constrains the frequency response and accuracy of ECG signal acquisition. To address this limitation, ongoing research focuses on optimizing OECT architectures and material systems to enhance response characteristics and improve their suitability for wearable ECG monitoring. For instance, Chen et al.[64] developed a fiber-based vertical OECT featuring a novel vertical channel structure with a narrow channel length of 3 μm. This design enables rapid response (12 ms), high maximum transconductance (16 mS), and good biocompatibility. The devices demonstrated stable, high-quality ECG signal recording with a SNR greater than 20.6 dB in both in vitro and in vivo environments (Table 1), highlighting their potential for long-term cardiac monitoring. Additionally, Zhang et al.[102] developed a flexible OECT array incorporating zwitterionic materials as the ionic electrolyte layer. The zwitterionic electrolyte facilitates rapid ion transport at the electrode interface, enhancing response speed and electrical performance. This design ensures reliable skin contact and stable ECG monitoring with high signal quality and a SNR of 20 dB.
4.4 Integrated flexible electronics
Through advancements in electronic materials and micro-nano fabrication technologies, recent developments have yielded miniaturized ECG monitoring systems that integrate signal acquisition, processing, and display functionalities[47]. Integrated flexible electronics enable conformal interfacing with skin surfaces, facilitating real-time monitoring of diverse physiological signals such as cardiac activity, respiratory rate, and blood pressure[70].
Electronic skin (E-skin) is an innovative flexible wearable integrated device characterized by skin-like softness and ultrathinness, mimicking the functions of human skin[103]. E-skin demonstrates significant application potential in real-time continuous monitoring of vital signs such as ECG signals, EMG, and heart rate[104]. Current research focuses on designing novel e-skin devices with improved interfacial adhesion, breathability, and mechanical robustness to enhance physiological data acquisition and user experience. For instance, Guo et al.[105] developed an e-skin with a web-droplet-like architecture and adhesion mechanism, combining high breathability, strong skin-contact adhesion, and exceptional electrode conductivity. By mimicking spiderweb structures, this e-skin achieved robust adhesion through PDMS distribution and utilized semi-liquid metal materials to attain ultrahigh conductivity
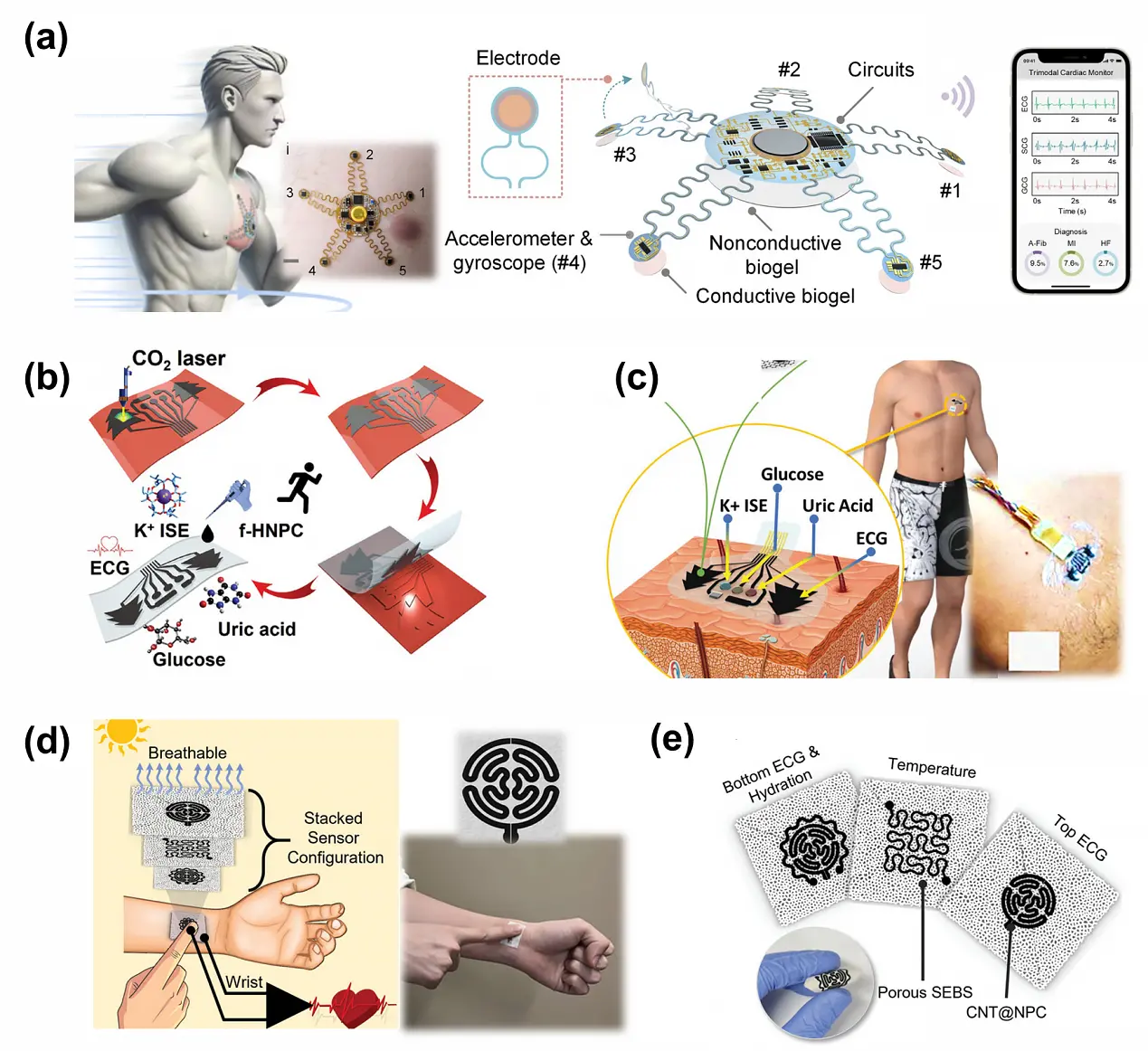
Figure 9. Flexible e-skin for ECG signal detection. (a) A starfish-like device for trimodal cardiac monitoring during motion[65]; (b), (c) Fabrication of the f-HNPC/LSG/SEBS-based epidermal sensor patch and its application for wearable detection of glucose, uric acid, K, and ECG pattern. Republished with permission from[9]; (d) A breathable e-skin for monitoring of temperature, hydration, and ECG. Republished with permission from[15]; (e) The breathable electrode design by depositing CNT@NPC as the sensing element on the porous SEBS substrate. Republished with permission from[15]. ECG: electrocardiograph; CNT: carbon nanotube; NPC: nanoporous carbon.
Multifunctional integrated electronics represent a significant development trend in wearable devices. To achieve more comprehensive health monitoring, various sensors are incorporated alongside ECG electrodes to detect multiple physiological parameters. For instance, a multifunctional electronic skin patch integrating an electrochemical sensor with a flexible skin electrode has been developed to enable simultaneous monitoring of sweat biomarkers and ECG signals (Figure 9b and Figure 9c)[9]. For sweat biomarker detection, a hybrid nanoporous graphene electrode array is constructed in situ on the substrate using a laser-scribed technique. The electrodes are individually functionalized with glucose oxidase, uricase, and a valinomycin-based potassium
5. Challenges and Perspective of Epidermal Electrodes for Wearable ECG Monitoring
5.1 Challenges
Recent advancements in flexible conductive materials and stretchable electrode architectures have substantially improved epidermal compatibility and signal acquisition stability, enabling long-term health monitoring through conformal skin contact. However, several challenges persist, particularly in the areas of dynamic signal fidelity, energy integration, and wearability. One major challenge in wearable ECG monitoring is the maintenance of signal accuracy under motion. Noise interference caused by body movement or skin deformation complicates ECG signal acquisition during physical activities, hindering accurate physiological data interpretation and raising concerns about clinical validation[108]. Therefore, the development of wearable ECG electrodes that exhibit low susceptibility to motion artifacts and provide high-quality signal acquisition under dynamic conditions remains a demanding objective[92]. Regarding energy integration, transparent conductive electrodes often encounter a trade-off between electrical conductivity and mechanical flexibility when combined with energy harvesting components. To support extended real-time monitoring and continuous data transmission, these electrodes must be equipped with optimized power management systems, efficient data compression techniques, and reliable communication protocols. Although environmental energy harvesting presents a promising approach, the efficient conversion and storage of harvested energy remain technical hurdles. Furthermore, optimizing power distribution for systems that integrate multiple sensors with varying power requirements poses a significant design challenge. Future efforts are expected to focus on improving the efficiency of energy harvesting, minimizing power consumption, and extending battery life, all without compromising device performance[109,110]. In terms of wearability, current flexible electrodes often exhibit limited breathability, which becomes especially problematic during sweat monitoring, where electrochemical sensors require close and continuous skin contact. As a result, the development of electrodes that concurrently offer breathability, flexibility, stretchability, and comfort remains a critical direction for future research and technological advancement[111].
5.2 Future perspective
The development of epidermal ECG electrodes is progressing toward multifunctional and intelligent systems that incorporate artificial intelligence (AI), advanced materials, and energy-efficient designs. AI-driven analytics are anticipated to play a transformative role in enhancing the diagnostic capabilities of wearable ECG monitors. Deep learning algorithms trained on
The integration of Internet of Things technologies will further expand the application scope of epidermal electrodes in telemedicine. Wireless communication protocols can enable seamless data transmission between wearable sensors, mobile devices, and
Material innovations are expected to resolve persistent challenges related to environmental adaptability and user comfort. Bioinspired adhesive designs, such as gecko-like hierarchical microstructures, may provide reliable skin attachment during dynamic movements without the use of irritating adhesives[118]. Environmentally resilient hydrogels with tunable properties, including antifreeze networks and moisture-retentive polymers, could help maintain stable electrical conductivity under extreme temperature or humidity conditions[51]. In addition, self-healing conductive polymers or nanocomposites containing surface chemical or physical binding groups are anticipated to improve mechanical durability, enabling electrodes to endure repeated deformation while reducing motion-induced artifacts[119].
To support real-world adoption, future research should prioritize scalable manufacturing methods and comprehensive clinical validation. The development of standardized testing protocols to evaluate long-term biocompatibility, signal stability, and compatibility with healthcare systems will be essential. By overcoming these challenges, next-generation epidermal electrodes have the potential to evolve from consumer-grade fitness devices into clinically validated diagnostic tools, thereby expanding access to proactive cardiovascular care and contributing to the transformation of global healthcare practices.
Authors contributions
Ji J: Writing-original draft.
Su T, Lu J, Gao XL: Investigation & visualization.
Zhang L: Writing-review & editing.
Conflicts of interest
Lei Zhang is an Editorial Board member of the BME Horizon. The other author declares no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was financially supported by a grant from Zhejiang Province Vanguard Goose-Leading Initiative (2024C04040, 2025C01072), the Fundamental Research Funds for the Central Universities of China (No. 226-2022-00083).
Copyright
© The Author(s) 2025.
References
-
1. Uvarov NF, Bokhonov BB, Ulihin AS, Titkov AI, Yukhin YM, Avdeeva DK, et al. Application of composite distributed electrodes in cardiographic sensors. Micro Nanosyst. 2021;13(4):426-432.
[DOI] -
2. Yang A, Song J, Liu H, Zhao Z, Li L, Yan F. Wearable organic electrochemical transistor array for skin‐surface electrocardiogram mapping above a human heart. Adv Funct Mater. 2023;33(17):2215037.
[DOI] -
3. Xi Y, Cheng S, Chao S, Hu Y, Cai M, Zou Y, et al. Piezoelectric wearable atrial fibrillation prediction wristband enabled by machine learning and hydrogel affinity. Nano Res. 2023;16:11674-11681.
[DOI] -
4. Lindstrom M, DeCleene N, Dorsey H, Fuster V, Johnson CO, LeGrand KE, et al. Global burden of cardiovascular diseases and risks collaboration, 1990-2021. JACC. 2022;80(25):2372-2425.
[DOI] -
5. Su B, Li J, Xu H, Xu Z, Meng J, Chen X, et al. Scientific athletics training: flexible sensors and wearable devices forkineses monitoring applications. Sci Sin Inf. 2022;52:54-74.
[DOI] -
6. Luo J, Sun C, Chang B, Zhang B, Li K, Li Y, et al. On‐skin paintable water‐resistant biohydrogel for wearable bioelectronics. Adv Funct Mater. 2024;34(34):2400884.
[DOI] -
7. Zhang S, Chhetry A, Zahed MA, Sharma S, Park C, Yoon S, et al. On-skin ultrathin and stretchable multifunctional sensor for smart healthcare wearables. npj Flex Electron. 2022;6:11.
[DOI] -
8. Kim JH, Kim SR, Kil HJ, Kim YC, Park JW. Highly conformable, transparent electrodes for epidermal electronics. Nano Lett. 2018;18(7):4531-4540.
[DOI] -
9. Asaduzzaman M, Faruk O, Samad AA, Kim H, Reza MS, Lee Y, et al. A MOFs-derived hydroxyl-functionalized hybrid nanoporous carbon incorporated laser-scribed graphene-based multimodal skin patch for perspiration analysis and electrocardiogram monitoring. Adv Funct Mater. 2024;34(40):2405651.
[DOI] -
10. Wang W, Zhou H, Xu Z, Li Z, Zhang L, Wan P. Flexible conformally bioadhesive MXene hydrogel electronics for machine learning-facilitated human-interactive sensing. Adv Mater. 2024;36(31):2401035.
[DOI] -
11. Kim S, Park YG, Kim JY, Kim E, Lee DH, Lee JH, et al. Magnetic manipulation of locomotive liquid electrodes for wireless active cardiac monitoring. ACS Appl Mater Interfaces. 2023;15(24):28954-28963.
[DOI] -
12. Shin JH, Choi JY, June K, Choi H, Kim T. Polymeric conductive adhesive-based ultrathin epidermal electrodes for long-term monitoring of electrophysiological signals. Adv Mater. 2024;36(23):2313157.
[DOI] -
13. Hu Z, Gu P, Yang X, Sun Z, Lu L, Liang X, et al. Nanoporous conjugated polymer aerogel films for high-performance electrochemical transistors. Adv Funct Mater. 2024;34(52):2410788.
[DOI] -
14. Lee I, Kim JH, Kim Y, Shin D, Lee H, Won J, et al. Ultraflexible vertical corbino organic electrochemical transistors for epidermal signal monitoring. Adv Mater. 2025;37(4):2410444.
[DOI] -
15. Pradhan GB, Jeong S, Sharma S, Lim S, Shrestha K, Lee Y, et al. A breathable and strain-insensitive multi-layered e-skin patch for digital healthcare wearables. Adv Funct Mater. 2024;34(46):2407978.
[DOI] -
16. Ma W. Research progress on the application of digital electrocardiographs. Chin Med Device Inf. 2024;30:68-70+160. Chinese.
[DOI] -
17. Ren X, Zhang J, Zhang M. Research progress on the application of remote ECG monitoring technology in patients with coronary heart disease. Chin Med Devices. 2024;39(01):144-149. Chinese. Available from: https://kns.cnki.net/kcms2/article/abstract?v=9IId9Ku_yBZ3StK7wMjlUbyfBO0-zocZqJyFnNS-1cSBDtFI94wbGRGTAxriTusRtGxmqiIc_3avB6kCOEPkmA4puJz62HPENLQtvNpgqnQco28B6EODY5bM1AZWhtcHbHEvMEtn-eibOu9zQ==&uniplatform=NZKPT&language=CHS
-
18. Salinet JL, Luppi Silva O. Chapter 2 - ECG signal acquisition systems. In: do Vale Madeiro JP, Cortez PC, da Silva Monteiro Filho JM, Brayner ARA, editors. Developments and Applications for ECG Signal Processing. San Diego: Academic Press; 2019. p. 29-51.
[DOI] -
19. Liu C, Yang M, Di J, Xing Y, Li Y, Li J. Wearable ECG: history, key technologies and future challenges. Chin J Biomed Eng. 2019;38(06):641-652. Chinese. Available from: https://kns.cnki.net/kcms2/article/abstract?v=9IId9Ku_yBbr1DPsyFPnwbo7eeok41tTGrDowZKML5lR-GR9IJiGPpkCRgXNfC1uPOmOC5qk4YdRynThPuT2-c9WEA7qAV_Utm41TvWPAEg8WFgi3WPy3Q6VRO-b2oymZ0nfFmrz0zQPeUEuajm0eYAg==&uniplatform=NZKPT&language=CHS
-
20. Liu H, Wang L, Niu X, Li H. Preparation and characterization of double-layer spacer fabric dry electrodes for ECG tracking. J Tiangong Univ. 2024;43(01):56-63. Chinese. Available from: https://kns.cnki.net/kcms2/article/abstract?v=9IId9Ku_yBZuxp2L7-u1fpYb_Yus-rToZJUsyYYl7J9-m12bHlmWSIpf_0Rzt5s1KDktZXk3viBqSLd4QT-x9_zQGf0rQujv9ixPJ6yL7rGIjOyi4gbyty-NdiBmGofcOWTpstiooI-UQ7XMFMP1qww==&uniplatform=NZKPT&language=CHS
-
21. Rattfält L. Smartware electrodes for ECG measurements: design, evaluation and signal processing. Sweden: Linkopings Universitet; 2013.
-
22. Rong L, Xie X, Yuan W, Fu Y. Superior, environmentally tolerant, flexible, and adhesive poly(ionic liquid) gel as a multifaceted underwater sensor. ACS Appl Mater Interfaces. 2022;14(25):29273-29283.
[DOI] -
23. Ding Y, Shi Y, Cheng H, Yu D, Wang W. A hydrophobic graft-modified PVA hydrogel by michael addition joint with gelation strategy for underwater cardiac sensing. Polymer. 2024;308:127369.
[DOI] -
24. Modoudi Yaghouti A, Jahanshahi A. Advancing wearable bioelectronics in emerging applications: seamless ECG monitoring through mechanically-supported electrical interconnects. Flex Print Electron. 2025;10:025001.
[DOI] -
25. Gao J, Hu M, Sun H, Wang Y, Wei Y, Li W, et al. Disposable and flexible smart electronic tapes for long-term biopotential monitoring. npj Flex Electron. 2025;9:6.
[DOI] -
26. Wang H, Wang J, Chen D, Ge S, Liu Y, Wang Z, et al. Robust tattoo electrode prepared by paper-assisted water transfer printing for wearable health monitoring. IEEE Sens J. 2022;22:3817-3827.
[DOI] -
27. Niu X, Wang L, Li H, Wang T, Liu H, He Y. Fructus xanthii-inspired low dynamic noise dry bioelectrodes for surface monitoring of ECG. ACS Appl Mater Interfaces. 2022;14(4):6028-6038.
[DOI] -
28. Niu X, Gao X, Wang T, Wang W, Liu H. Ordered nanopillar arrays of low dynamic noise dry bioelectrodes for electrocardiogram surface monitoring. ACS Appl Mater Interfaces. 2022;14(29):33861-33870.
[DOI] -
29. Tian Q, Zhao H, Wang X, Jiang Y, Zhu M, Yelemulati H, et al. Hairy-skin-adaptive viscoelastic dry electrodes for long-term electrophysiological monitoring. Adv Mater. 2023;35(30):2211236.
[DOI] -
30. Zhang Y, Zhou J, Zhang Y, Zhang D, Yong KT, Xiong J. Elastic fibers/fabrics for wearables and bioelectronics. Adv Sci. 2022;9(35):2203808.
[DOI] -
31. Niu L, Shen Z, Wang Z, Qi L, Niu H, Zhou H, et al. Low-contact impedance textile electrode for real-time detection of ECG signals. ACS Appl Mater Interfaces. 2024;16(42):57860-57869.
[DOI] -
32. Heredia-Rivera U, Krishnakumar A, Kasi V, Rana MM, Gopalakrishnan S, Nejati S, et al. Cold atmospheric plasma deposition of antibacterial polypyrrole-silver nanocomposites on wearable electronics for prolonged performance. J Mater Chem C. 2024;12(31):11861-11876.
[DOI] -
33. Le K, Soltanian S, Narayana H, Servati A, Servati P, Ko F. Roll-to-roll fabrication of silver/silver chloride coated yarns for dry electrodes and applications in biosignal monitoring. Sci Rep. 2023;13:21182.
[DOI] -
34. Sadri B, Gao W. Fibrous wearable and implantable bioelectronics. Appl Phys Rev. 2023;10:031303.
[DOI] -
35. Guo R, Li T, Jiang C, Zong H, Li X, Wan C, et al. Pressure regulated printing of semiliquid metal on electrospinning film enables breathable and waterproof wearable electronics. Adv Fiber Mater. 2024;6:354-366.
[DOI] -
36. Yang X, Wang S, Liu M, Li L, Zhao Y, Wang Y, et al. All-nanofiber-based janus epidermal electrode with directional sweat permeability for artifact-free biopotential monitoring. Small. 2022;18(12):2106477.
[DOI] -
37. Eskandarian L, Toossi A, Nassif F, Golmohammadi Rostami S, Ni S, Mahnam A, et al. 3D-Knit dry electrodes using conductive elastomeric fibers for long-term continuous electrophysiological monitoring. Adv Mater Technol. 2022;7(7):2101572.
[DOI] -
38. Ratner BD, Schoen FJ. 2.3.2 - The concept and assessment of biocompatibility. In: Wagner WR, Sakiyama-Elbert SE, Zhang G, Yaszemski MJ, editors. Biomaterials Science. San Diego: Academic Press; 2020. p. 843-849.
[DOI] -
39. Spector M. 4.1 The Concept of Biocompatibility. In: DucheyneP, editor. Comprehensive Biomaterials II. The Netherlands: Elsevier Science Ltd; 2017. p. 1-6.
[DOI] -
40. Hu X, Wang C, Huang J, Yan Y, Liao X, Li Y, et al. Self-adhesive epidermal bioelectrodes for long-term electrophysiological monitoring and emotion recognition. Adv Mater Technol. 2025;10(10):2401800.
[DOI] -
41. Stojanović GM, Popović Ž, Milić L, Simić M. Potato peels-based electrodes for recording ECG and EMG signals. Sens Bio-Sens Res. 2024;44:100664.
[DOI] -
42. Yan X, Chen S, Zhang G, Shi W, Peng Z, Liu Z, et al. Highly breathable, surface-hydrophobic and wet-adhesive silk based epidermal electrode for long-term electrophysiological monitoring. Compos Sci Technol. 2022;230:109751.
[DOI] -
43. Owda AY, Casson AJ. A mini-review of graphene based materials for electrodes in electrocardiogram (ECG) sensing. In: 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS); 2021 Jun 20-23; Manchester, United Kingdom. New York: IEEE; 2021; 1-4.
[DOI] -
44. Hua Q, Shen G. Low-dimensional nanostructures for monolithic 3D-integrated flexible and stretchable electronics. Chem Soc Rev. 2024;53(3):1316-1353.
[DOI] -
45. Park S, Heo SW, Lee W, Inoue D, Jiang Z, Yu K, et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature. 2018;561:516-521.
[DOI] -
46. Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, et al. Epidermal electronics. Science. 2011;333(6044):838-843.
[DOI] -
47. Wang K, Parekh U, Pailla T, Garudadri H, Gilja V, Ng TN. Stretchable dry electrodes with concentric ring geometry for enhancing spatial resolution in electrophysiology. Adv Healthc Mater. 2017;6(19):1700552.
[DOI] -
48. Bhattacharya S, Nikbakht M, Alden A, Tan P, Wang J, Alhalimi TA, et al. A chest-conformable, wireless electro-mechanical e-tattoo for measuring multiple cardiac time intervals. Adv Electron Mater. 2023;9(9):2201284.
[DOI] -
49. Meziane N, Webster JG, Attari M, Nimunkar AJ. Dry electrodes for electrocardiography. Physiol Meas. 2013;34:R47.
[DOI] -
50. Ding H, Gu Y, Ren Y, Hu C, Qiu Q, Wu D, et al. The latest research progress of conductive hydrogels in the field of electrophysiological signal acquisition. J Mater. Chem C. 2024;12(9):3030-3052.
[DOI] -
51. Li J, Ban Q, Xu M, Wang S, Geng J, Zhang Z, et al. Tissue-adhesive, silk-based conductive hydrogel with high stretchable, transparent, healable and degradable properties for real-time, precise monitoring of tissue motions and electrocardiogram under sweaty condition. J Colloid Interface Sci. 2025;691:137455.
[DOI] -
52. You L, Zheng Z, Xu W, Wang Y, Xiong W, Xiong C, et al. Self-healing and adhesive MXene-polypyrrole/silk fibroin/polyvinyl alcohol conductive hydrogels as wearable sensor. Int J Biol Macromol. 2024;263:130439.
[DOI] -
53. Vurro D, Liboà A, D’Onofrio I, De Giorgio G, Scaravonati S, Crepaldi M, et al. Sericin electrodes with self-adhesive properties for biosignaling. ACS Biomater Sci Eng. 2025;11(3):1776-1791.
[DOI] -
54. Li X, Sun Y, Wang S, Tian G, Yang T, Huang L, et al. Body temperature-triggered adhesive ionic conductive hydrogels for bioelectrical signal monitoring. Chem Eng J. 2024;498:155195.
[DOI] -
55. Ahmed F, Song J, Masud , Lee S, Kim J. Advances in the development of hydrogel-based adhesion layers for wearable health monitors: focusing on biocompatibility, conductivity, and mechanical strength. ACS Appl Polym Mater. 2024;6(22):13497-13511.
[DOI] -
56. Tang S, Sha D, He Z, Chen X, Ma Y, Liu C, et al. Environmentally adaptable organo-ionic gel-based electrodes for real-time on-skin electrocardiography monitoring. Adv Healthc Mater. 2023;12(18):2300475.
[DOI] -
57. Yao S, Zhang C, Bai L, Wang S, Liu Y, Li L, et al. Tailoring stretchable, biocompatible, and 3D printable properties of carbon-based conductive hydrogel for bioelectronic interface applications. Adv Funct Mater. 2025;35(12):2418554.
[DOI] -
58. Wang P, Lv Y, Duan J, Sun G, Meng C, Li Y, et al. A thermally responsive phase-change hydrogel for skin-mountable multifunctional sensors. Nano Energy. 2025;136:110722.
[DOI] -
59. He H, Chen Y, Pu A, Wang L, Li W, Zhou X, et al. Strong and high-conductivity hydrogels with all-polymer nanofibrous networks for applications as high-capacitance flexible electrodes. npj Flex Electron. 2024;8:56.
[DOI] -
60. Xu Y, Su Y, Xu X, Arends B, Zhao G, Ackerman DN, et al. Porous liquid metal-elastomer composites with high leakage resistance and antimicrobial property for skin-interfaced bioelectronics. Sci Adv. 2023;9(1):eadf0575.
[DOI] -
61. Yu Z, Wu P. Underwater communication and optical camouflage ionogels. Adv Mater. 2021;33(24):2008479.
[DOI] -
62. Lan L, Ping J, Li H, Wang C, Li G, Song J, et al. Skin-inspired all-natural biogel for bioadhesive interface. Adv Mater. 2024;36(25):2401151.
[DOI] -
63. Shi Y, Ding Y, Wang W, Yu D. High-performance, water-resistant and ion-conducting gel used as underwater ECG electrodes. J Mater Sci. 2023;58:16415-16427.
[DOI] -
64. Kim W, Bang J, Yang Y, Ko TH, Jang M, Cha JJ, et al. Highly stretchable and conductive kirigami-like double-layer electrodes for motion-insensitive wearable electronics. Compos Part B Eng. 2024;283:111655.
[DOI] -
65. Cui T, Qiao Y, Li D, Huang X, Yang L, Yan A, et al. Multifunctional, breathable MXene-PU mesh electronic skin for wearable intelligent 12-lead ECG monitoring system. Chem Eng J. 2023;455:140690.
[DOI] -
66. Lin X, Ou Z, Wang X, Wang C, Ouyang Y, Mwakitawa IM, et al. Self‐adhesive and biocompatible dry electrodes with conformal contact to skin for epidermal electrophysiology. Interdiscip Mater. 2024;3(5):775-790.
[DOI] -
67. Chen J, Fang Y, Feng J, Shi X, Li J, Wang S, et al. Fast-response fiber organic electrochemical transistor with vertical channel design for electrophysiological monitoring. J Mater Chem B. 2024;12(37):9206-9212.
[DOI] -
68. Chen S, Ouyang Q, Meng X, Yang Y, Li C, Miao X, et al. Starfish-inspired wearable bioelectronic systems for physiological signal monitoring during motion and real-time heart disease diagnosis. Sci Adv. 2025;11(14):eadv2406.
[DOI] -
69. Chung HU, Kim BH, Lee JY, Lee J, Xie Z, Ibler EM, et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science. 2019;363(6430):eaau0780.
[DOI] -
70. Yang S, Liu C, Tang L, Shang J, Zhang J, Jiang X. Highly adhesive and stretchable epidermal electrode for bimodal recording patch. ACS Appl Mater Interfaces. 2024;16(33):43880-43891.
[DOI] -
71. Alex M, Khan KRB, Al-Othman A, Al-Sayah MH, Al Nashash H. MXene-based flexible electrodes for electrophysiological monitoring. Sensors. 2024;24(11):3260.
[DOI] -
72. Tu H, Xu T, Xuan F, Gao Y, Yang H. Effect of silver chloride content on the performance of fully printed fabric electrodes for ECG monitoring. J Sens. 2024;2024:8839634.
[DOI] -
73. Maithani Y, Singh A, Mehta BR, Singh JP. PEDOT: PSS treated cotton-based textile dry electrode for ECG sensing. Mater Today Proc. 2022;62:4052-4057.
[DOI] -
74. Shih M, Kuo CT, Lin MH, Chuang YJ, Chen H, Yew TR. A 3D-CNT micro-electrode array for zebrafish ECG study including directionality measurement and drug test. Biocybern Biomed Eng. 2020;40(2):701-708.
[DOI] -
75. Wu Q, Chen A, Chen Y, Zhang J, Han S, Huang J, et al. A Stretchable and tough conductive elastic film for multifunctional flexible strain gauges. Adv Sens Res. 2024;3(6):2300208.
[DOI] -
76. Hilbel T, Frey N. Review of current ECG consumer electronics (pros and cons). J Electrocardiol. 2023;77:23-28.
[DOI] -
77. Heo RH, Foroutan F, De Luca E, Albertini L, Rac VE, Cafazzo JA, et al. Feasibility of single-lead apple watch electrocardiogram in atrial fibrillation detection among heart failure patients. JACC Adv. 2024;3:101051.
[DOI] -
78. Butler L, Ivanov A, Celik T, Karabayir I, Chinthala L, Hudson MM, et al. Feasibility of remote monitoring for fatal coronary heart disease using Apple Watch ECGs. Cardiovasc Digit Health J. 2024;5(3):115-121.
[DOI] -
79. Sharma S, Chhetry A, Jeong S, Park JY. A strain-insensitive stretchable patch sensor for simultaneous monitoring of body temperature and ECG. In: 2023 22nd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers); 2023 Jun 25-29; Kyoto, Japan. Piscataway: IEEE; 2023. p. 310-313.
-
80. Zahed MA, Kim DK, Jeong SH, Selim Reza M, Sharifuzzaman M, Pradhan GB, et al. Microfluidic-integrated multimodal wearable hybrid patch for wireless and continuous physiological monitoring. ACS Sens. 2023;8(8):2960-2974.
[DOI] -
81. Fan JA, Yeo W-H, Su Y, Hattori Y, Lee W, Jung SY, et al. Fractal design concepts for stretchable electronics. Nat Commun. 2014;5:3266.
[DOI] -
82. Liu G, Xu C, Ren J, Liu Y, Yao L, Xie Y, et al. Embossing micro-fabrication-based epidermal electrode with a CNT-composed metal film as human-machine interface. J Mater Sci Mater Electron. 2023;34:1254.
[DOI] -
83. Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nature Mater. 2015;14:23-36.
[DOI] -
84. Yang L, Hu Z, Xiang Z, Zhou J, Wang X, Liu Q, et al. A high-entropy electrode material for electrobiochemical and eletrophysiological signals detection. Chem Eng J. 2024;499:156209.
[DOI] -
85. Gwak H, Cho S, Song YJ, Park JH, Seo S. A study on the fabrication of metal microneedle array electrodes for ECG detection based on low melting point Bi-In-Sn alloys. Sci Rep. 2023;13:22931.
[DOI] -
86. Ping B, Zhang Z, Liu Q, Li M, Yang Q, Guo R. Liquid metal fibers with a knitted structure for wearable electronics. Biosensors. 2023;13:715.
[DOI] -
87. Liu H, Peng Y, Sun J, Zhang Y, Long J, Gu Y, et al. Proanthocyanidin-enhanced wettability and adhesion in liquid metal inks for multi-substrate patterning in soft electronics. J Mater Chem A. 2025;13(2):1302-1312.
[DOI] -
88. Qu M, Zhu M, Lv Y, Liu Q, Li J, Gao Y, et al. Hydrophobic TPU/CNTs-ILs ionogel as a reliable multimode and flexible wearable sensor for motion monitoring, information transfer, and underwater sensing. ACS Appl Mater Interfaces. 2024;16:35626-35638.
[DOI] -
89. Xu X, Li Z, Hu M, Zhao W, Dong S, Sun J, et al. High sensitivity and antifreeze silver nanowire/eutectic gel strain sensor for human motion and healthcare monitoring. IEEE Sens J. 2024;24(5):5928-5935.
[DOI] -
90. Harimurti S, Wang W, Sasaki K, Okuda C, Jonathan Wijaya T, Osman Goni Nayeem M, et al. Janus electrode with stable asymmetric wettability for robust biosignal monitoring on sweaty skin. Mater Today. 2024;74:94-108.
[DOI] -
91. Park M, Yang W, Kim JW, Choi Y, Kim S, Lee Y, et al. A fully self-healing patch of integrated bio-signal monitoring sensors with self-healing microporous foam and Au nanosheet electrodes. Adv Funct Mater. 2024;34(38):2402508.
[DOI] -
92. Kang TW, Lee J, Kwon Y, Lee YJ, Yeo WH. Recent progress in the development of flexible wearable electrodes for electrocardiogram monitoring during exercise. Adv NanoBiomed Res. 2024;4(8):2300169.
[DOI] -
93. Gullo L, Mazzaracchio V, Colozza N, Duranti L, Fiore L, Arduini F. Carbon black as a cost-effective nanomodifier for (electro)chemical-free pre-treatment thermoplastic polyurethane-based 3D printed electrodes. Electrochim Acta. 2024;482:143982.
[DOI] -
94. Aung HH, Qi Z, Niu Y, Guo Y. Rapid production of carbon nanotube film for bioelectronic applications. Nanomaterials. 2023;13(11):1749.
[DOI] -
95. Wang L, Zhang F, Su W, Xu X, Li A, Li Y, et al. Green manufacturing of flexible sensors with a giant gauge factor: bridging effect of CNT and electric field enhancement at the percolation threshold. ACS Appl Mater Interfaces. 2022;14(22):26024-26033.
[DOI] -
96. Gao X, Bao Y, Chen Z, Lu J, Su T, Zhang L, et al. Bioelectronic applications of intrinsically conductive polymers. Adv Electron Mater. 2023;9(10):2300082.
[DOI] -
97. Du X, Yang L, Liu N. Recent Progress on poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) bioelectrodes. Small Sci. 2023;3(7):2300008.
[DOI] -
98. Lu D, Chen H. Solid-state organic electrochemical transistors (OECTs) based on gel electrolytes for biosensors and bioelectronics. J Mater Chem A. 2024;13(1):136-157.
[DOI] -
99. Rivnay J, Inal S, Salleo A, Owens RM, Berggren M, Malliaras GG. Organic electrochemical transistors. Nat Rev Mater. 2018;3:17086.
[DOI] -
100. Yu S, Sun X, Liu J, Li S. OECT - inspired electrical detection. Talanta. 2024;275:126180.
[DOI] -
101. Jiang X, Liang Z, Wu M, Lu J, Shi C, Wang Q, et al. High-performance organic electrochemical transistors gated with 3D-printed graphene oxide electrodes. Nano Res. 2023;16:12689-12696.
[DOI] -
102. Zhang S, Qian SH, Zhao G, Pan QC, Song R, Zhang T, et al. Intrinsically antifouling, soft and conformal bioelectronic from scalable fabrication of Thin-Film OECT arrays by zwitterionic polymers. Chem Eng J. 2024;483:148980.
[DOI] -
103. Chen Y, Gao Z, Zhang F, Wen Z, Sun X. Recent progress in self-powered multifunctional e-skin for advanced applications. Exploration. 2022;2(1):20210112.
[DOI] -
104. Liu X, Li H, Tao M, Yu Y, Zhu Z, Wu D, et al. Organic flexible electronics for innovative applications in electronic skin. Adv Mater Technol. 2025;10(3):2400661.
[DOI] -
105. Guo R, Li X, Zhou Y, Zhang Y, Jiang C, Yu Y, et al. Semi-liquid metal-based highly permeable and adhesive electronic skin inspired by spider web. Sci Bull. 2024;69(17):2723-2734.
[DOI] -
106. Yang Y, Song B, Zhang J, Dan N, Gu H. Multifunctional, high-strength electronic skin based on the natural sheepskin fiber network for multifaceted human health monitoring and management. Biomacromolecules. 2024;25(8):5359-5373.
[DOI] -
107. Bhattacharya S, Santucci F, Jankovic M, Huang T, Basu J, Tan P, et al. Cardiac time intervals under motion using bimodal chest E-tattoos and multistage processing. IEEE Trans Biomed Eng. 2025;72:413-424.
[DOI] -
108. Umar AH, Othman MA, Harun FKC, Yusof Y. Dielectrics for non-contact ECG bioelectrodes: a review. IEEE Sens J. 2021;21(17):18353-18367.
[DOI] -
109. Ouyang H, Jiang D, Fan Y, Wang ZL, Li Z. Self-powered technology for next-generation biosensor. Sci Bull. 2021;66(17):1709-1712.
[DOI] -
110. Arya S, Sharma A, Singh A, Ahmed A, Dubey A, Padha B, et al. Review-energy and power requirements for wearable sensors. ECS Sens Plus. 2024;3:022601.
[DOI] -
111. Xue Z, Gai Y, Wu Y, Liu Z, Li Z. Wearable mechanical and electrochemical sensors for real-time health monitoring. Commun Mater. 2024;5:211.
[DOI] -
112. Li F, Wang P, Wang Li X. Deep learning-based regional ECG diagnosis platform. Pacing Clin Electrophysiol. 2024;47(1):139-148.
[DOI] -
113. Jameel GS, Al-Turfi MN, Al-Obaidi WS, Hamoudi GH. Portable ECG device based on deep learning and raspberry PI 4. Iraqia J Sci Eng Res. 2024;3(2):77-82.
[DOI] -
114. Seddiki M, Bouhedda M, Tobbal A, Rebouh S, Aniba Y. AI-Driven IoT healthcare system for real-time ECG analysis and comprehensive patient monitoring. Sci Eng Technol. 2024;4:61-81.
[DOI] -
115. Hozumi S, Honda S, Arie T, Akita S, Takei K. Multimodal wearable sensor sheet for health-related chemical and physical monitoring. ACS Sens. 2021;6(5):1918-1924.
[DOI] -
116. Hui X, Asaduzzaman M, Zahed MA, Sharma S, Jeong S, Song H, et al. Multifunctional siloxene-decorated laser-inscribed graphene patch for sweat ion analysis and electrocardiogram monitoring. ACS Appl Mater Interfaces. 2024;16(8):9725-9735.
[DOI] -
117. Wang Z, Liu M, Zhao Y, Chen Y, Noureen B, Du L, et al. Functional organic electrochemical transistor-based biosensors for biomedical applications. Chemosensors. 2024;12(11):236.
[DOI] -
118. Yang S, Jiang X. Nanoscale strategies for enhancing the performance of adhesive dry electrodes for the skin. ACS Nano. 2024;18(40):27107-27125.
[DOI] -
119. Lin Y, Wu A, Zhang Y, Duan H, Zhu P, Mao Y. Recent progress of nanomaterials-based composite hydrogel sensors for human-machine interactions. Discover Nano. 2025;20:60.
[DOI]
Copyright
© The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite