Table of Contents
The role of redoxins in ferroptosis
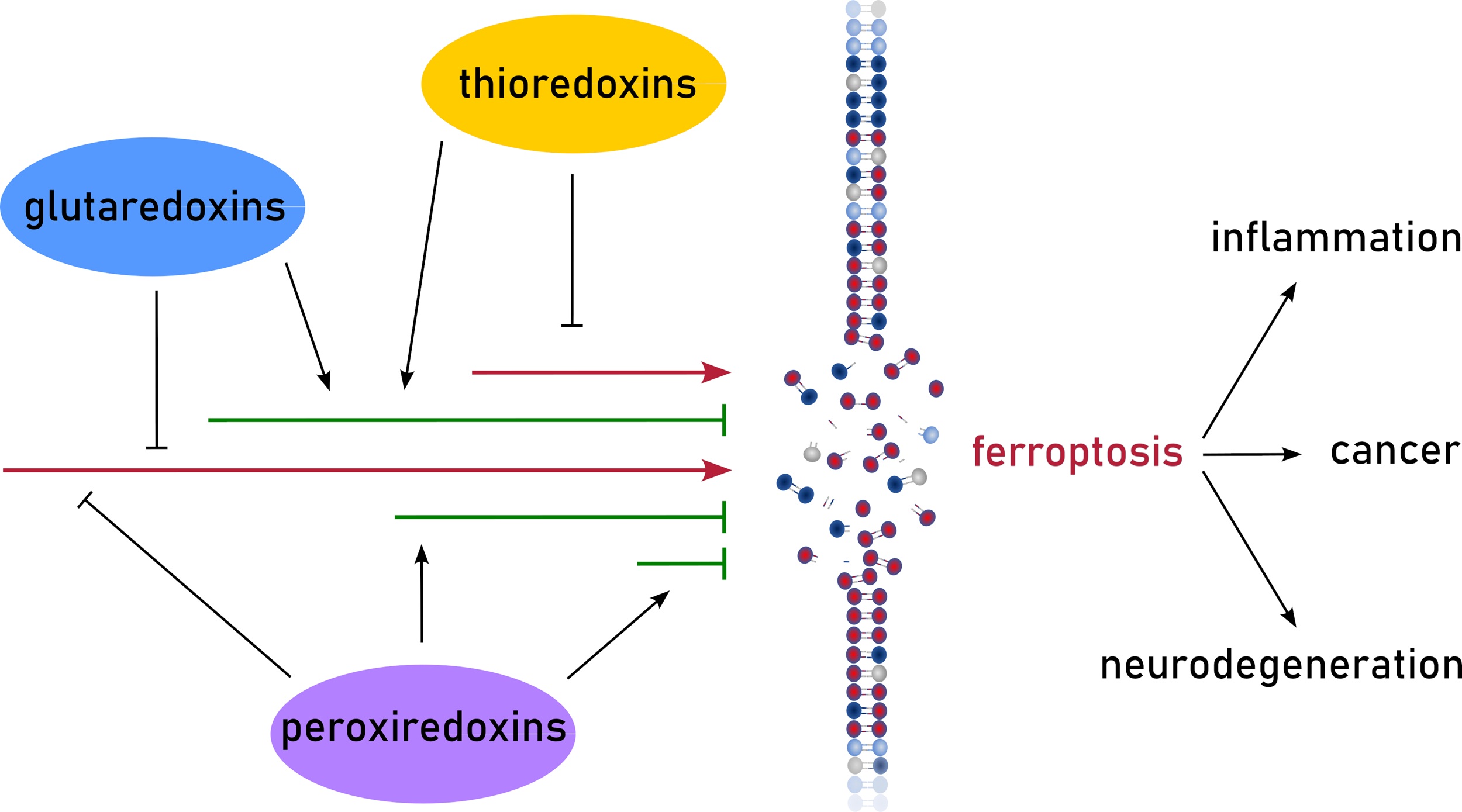
Redoxins, oxidoreductases of the thioredoxin (Trx) family, are important regulators of signaling processes. The Trx family is characterized by the Trx fold consisting of a four-stranded β-sheet surrounded by three-four α-helices. This article mainly focuses ...
More.Redoxins, oxidoreductases of the thioredoxin (Trx) family, are important regulators of signaling processes. The Trx family is characterized by the Trx fold consisting of a four-stranded β-sheet surrounded by three-four α-helices. This article mainly focuses on mammalian Trxs, glutaredoxins, and peroxiredoxins, herein referred to as redoxins. This review article summarizes the current knowledge on redoxin-driven processes related to ferroptosis, a non-apoptotic cell death mechanism based on uncontrolled oxidation of polyunsaturated fatty acids in membranes. To a great extent, the formation of these lipid hydroperoxides depend on the non-enzymatic formation of hydroxyl radicals, the product of the Fenton reaction between hydrogen peroxide and redox active iron. Redoxins are regulators of both redox and iron homeostasis, and some redoxins use lipid hydroperoxides directly as substrates. This review article aims to increase the recognition of redoxins as potential regulators of ferroptosis in both physiological and pathological conditions, while also promoting research to address the numerous gaps in cell specificity and the molecular mechanisms influencing ferroptotic pathways.
Less.Junya Ito, ... Carsten Berndt
DOI:https://doi.org/10.70401/fos.2025.0008 - December 22, 2025
Targeting mTORC1 to promote ferroptosis and apoptosis in endometrial cancer with PI3K-Akt-mTOR pathway mutation
Aims: Endometrial cancer (EC) is often driven by hyperactivation of the PI3K-Akt-mTOR (PAM) pathway due to mutations in PTEN and/or PI3K genes. While mechanistic target of rapamycin complex 1 (mTORC1) inhibitors show limited efficacy as single agents ...
More.Aims: Endometrial cancer (EC) is often driven by hyperactivation of the PI3K-Akt-mTOR (PAM) pathway due to mutations in PTEN and/or PI3K genes. While mechanistic target of rapamycin complex 1 (mTORC1) inhibitors show limited efficacy as single agents in EC, previous studies suggest that they may sensitize the PAM-mutant cancer cells to ferroptosis, a regulated form of necrosis dependent on iron-catalyzed lipid peroxidation. We investigated whether combining mTORC1 inhibition with ferroptosis induction could overcome resistance mechanisms and improve therapeutic outcomes in EC.
Methods: We evaluated the effect of catalytic, allosteric, and bi-steric mTORC1 inhibition on ferroptosis sensitivity in EC cell lines with different PAM pathway mutational statuses. In vivo efficacy of the combinational treatment was tested in MFE296 xenograft models.
Results: The catalytic and bi-steric mTORC1 inhibitor RMC-6272 sensitized PAM pathway-activated EC cells to ferroptosis induced by GPX4 inhibition, while EC cells without PAM pathway activation were intrinsically sensitive to ferroptosis. Further, mTORC1 inhibition also induced apoptosis in PAM pathway-activated EC cells, indicating a multi-modal cell death response. In vivo, combination treatment with RMC-6272 and the GPX4 inhibitor JKE-1674 significantly suppressed xenograft growth, with evidence of both ferroptosis and apoptosis in tumors.
Conclusion: Our study highlights the therapeutic potential of dual targeting of mTORC1 and ferroptosis to trigger multi-modal cell death in PAM pathway-activated EC, with broader implications for other cancers exhibiting mTORC1 hyperactivation.
Less.Yingying Hu, ... Xuejun Jiang
DOI:https://doi.org/10.70401/fos.2025.0005 - November 26, 2025
Disulfidptosis and its emerging relevance in cancer and immunity
Disulfidptosis is a recently identified form of regulated cell death (RCD) triggered by disulfide stress when cystine uptake via solute carrier family 7 member 1 (SLC7A11) overwhelms the cell’s reducing capacity. Unlike apoptosis or other “cell suicide” ...
More.Disulfidptosis is a recently identified form of regulated cell death (RCD) triggered by disulfide stress when cystine uptake via solute carrier family 7 member 1 (SLC7A11) overwhelms the cell’s reducing capacity. Unlike apoptosis or other “cell suicide” pathways, disulfidptosis likely represents a “cell sabotage” mechanism, defined by aberrant disulfide bonding and catastrophic actin cytoskeleton collapse. In this Perspective, we examine the paradoxical role of SLC7A11 as both a ferroptosis protector and a disulfidptosis trigger, and the mechanistic hallmarks of disulfidptosis. We highlight emerging therapeutic strategies to target disulfidptosis in cancer, including glucose transporter inhibition, redox-targeting agents, and nanomaterial-based approaches, and consider its dual role in immunity, where it may suppress T cell function yet act as a form of immunogenic cell death. Together, these insights position disulfidptosis as both a conceptual advance in RCD biology and a promising target for cancer therapy that warrants further mechanistic and translational exploration.
Less.Qidong Li, ... Boyi Gan
DOI:https://doi.org/10.70401/fos.2025.0004 - November 18, 2025
Lipidomic changes in persister cancer cells drive enhanced ferroptosis sensitivity
Aims: Unique in the broader category of drug-resistant cells, persister cancer cells (PSs) acquire their tolerance to compounds through reversible, chromatin-mediated changes, allowing them to ‘persist’ in the face of cancer therapeutic agents. ...
More.Aims: Unique in the broader category of drug-resistant cells, persister cancer cells (PSs) acquire their tolerance to compounds through reversible, chromatin-mediated changes, allowing them to ‘persist’ in the face of cancer therapeutic agents. PSs are implicated in minimal residual disease from which cancer relapse occurs, and given their established sensitivity to ferroptosis, PSs present a critical point through which identification and targeting of drug-resistant cancers may be possible. Ferroptosis sensitivity in drug-resistant cancers may be caused by the attainment of the persister state, or it may merely be correlative with this state and due instead to extended inhibition of oncogenic signaling or the induction of chemotherapy stress. Nonetheless, ferroptosis sensitivity has emerged as a common phenotype across multiple PS and drug-resistant cancer cell types. Identifying biomarkers for and drivers of ferroptosis sensitivity in drug-resistant and PS cells is therefore a high priority.
Methods: We derived PS cells from the lung carcinoma cell line PC9 (PSPC9), performed transcriptomic analysis, and subsequently lipidomics on the PC9/PSPC9 system. Additionally, we reverted PSPC9 cells to the ferroptosis-resistant parental state (PC9PS -> PC9) and assessed the resulting lipid changes. We generated two additional PS-like cell models: PS-like prostate carcinoma (PSLNCaP) from LNCaP cells and PS-like fibrosarcoma (PSHT1080) from HT1080 cells, with lipidomics analysis. Finally, we performed a mitochondrial elimination assay and assessed its effect on ferroptosis sensitivity.
Results: We observed enrichment of lipid and sugar metabolism gene expression in PSPC9; lipidomics revealed enrichment within PSPC9 for ferroptosis-driving diPUFA phospholipids (diPUFA-PL), as well as polyunsaturated free fatty acids (PUFA FFAs). Upon PSPC9 reversion to the ferroptosis-resistant parental state (PC9PS -> PC9), this lipid signature reverted. The LNCaP and HT1080 PS-like models individually showed features consistent with PS, including an increased labile-iron pool, reversibility, and enhanced ferroptosis sensitivity, and had lipid features consistent with those in PSPC9. Finally, mitochondrial elimination partially abrogated ferroptosis sensitivity and altered the PS lipid profile.
Conclusion: In summary, lipidomic changes dependent on the presence of mitochondria are key to the ferroptosis sensitivity of drug-tolerant persister cancer cells.
Less.Eduard Reznik, ... Brent R. Stockwell
DOI:https://doi.org/10.70401/fos.2025.0003 - November 10, 2025