Abstract
Diabetic foot ulcers (DFUs) are a serious complication of diabetes and often result in amputation. Traditional wound care methods have limitations in addressing the complex pathophysiology of DFUs. Hydrogel dressings, a type of biomaterial, have emerged as promising candidates for treating DFUs due to their biocompatibility, ability to retain moisture, and potential to incorporate therapeutic agents. Hydrogels create a moist environment, promote cell migration, and reduce inflammation, thereby supporting wound healing. Incorporating bioactive molecules, such as growth factors and anti-inflammatory agents, can further enhance the effectiveness of hydrogels. Additionally, stem cells can be loaded into hydrogels to improve tissue regeneration and help modulate the wound microenvironment. Recent advancements in hydrogel technology have also led to the development of smart hydrogels that can respond to changes in wound conditions, such as glucose levels and pH. These intelligent dressings offer personalized care by delivering targeted treatments based on real-time wound data. This review explores the mechanisms behind DFU development, the role of hydrogels in wound healing, and recent progress in hydrogel technologies for personalized DFU care.
Keywords
1. Introduction
Diabetes mellitus is a prevalent chronic disease characterized by elevated blood glucose levels[1,2]. It arises either from insufficient insulin production or impaired insulin utilization, resulting in hyperglycemia. According to statistical projections, approximately 463 million individuals worldwide were affected by diabetes in 2019, with this number expected to rise to 700 million by 2034[2]. Diabetes mellitus can lead to a variety of complications, among which diabetic foot ulcers (DFUs) represent a serious concern. Studies estimate that between 19% and 34% of individuals with diabetes will develop DFUs during their lifetime. The International Diabetes Federation reports that approximately 9.1 to 26.1 million new DFU cases occur annually[3,4]. Poor healing of DFUs often results in chronic wounds, frequently requiring surgical interventions such as amputation. Consequently, DFUs are a leading cause of nontraumatic lower limb amputations.
Current clinical treatments for the management of DFU include pressure relief, debridement, revascularization, and antibiotic therapy[5]. However, these approaches do not guarantee complete wound healing. Therefore, there is a pressing need for novel alternatives that can promote faster and more comprehensive wound repair with fewer complications. In this regard, wound dressings have attracted significant research interest as a promising strategy for effective DFU treatment and management. An ideal wound dressing should possess the following essential characteristics: (i) maintaining a moist environment to stimulate tissue regeneration, (ii) preventing microbial invasion, (iii) providing adequate porosity to allow gas exchange, (iv) promoting cell proliferation and neovascularization, and (v) exhibiting biocompatibility[6].
Hydrogel-based wound dressings fulfill many of these essential criteria and have demonstrated promising outcomes in the treatment of DFU. The combined beneficial properties of hydrogels are often referred to as the “gel effect,” reflecting their ability to restore and enhance wound healing in diabetic patients. Hydrogels are crosslinked polymeric materials capable of absorbing substantial amounts of water or physiological fluids without dissolving. Based on the nature of their crosslinking, hydrogels can be classified as either physical or chemical[7]. Physical crosslinking involves transient junctions formed through polymer chain entanglements or non-covalent interactions such as hydrogen bonding, hydrophobic forces, or ionic interactions. In contrast, chemically crosslinked hydrogels possess permanent covalent crosslink junctions, rendering them insoluble in water. The three-dimensional network structure of hydrogels mimics the properties of soft tissue and allows for structural modifications to facilitate the effective loading and controlled release of therapeutic agents, thereby supporting enhanced wound healing.
Given the promising potential of hydrogels in wound healing, this review focuses on recent advances in hydrogel-based wound dressing materials for the treatment and management of DFU. The review covers the normal and diabetic wound healing processes, explores various types of hydrogel-based wound dressings, and discusses future perspectives in this field.
2. Normal Wound Healing Process
Normal wound healing is a complex, regulated cascade of events that ultimately lead to re-epithelialization and restoration of the injured tissue. This process unfolds through four distinct physiological phases: (i) hemostasis, (ii) inflammation, (iii) proliferation, and (iv) remodeling[8], as illustrated in Figure 1.
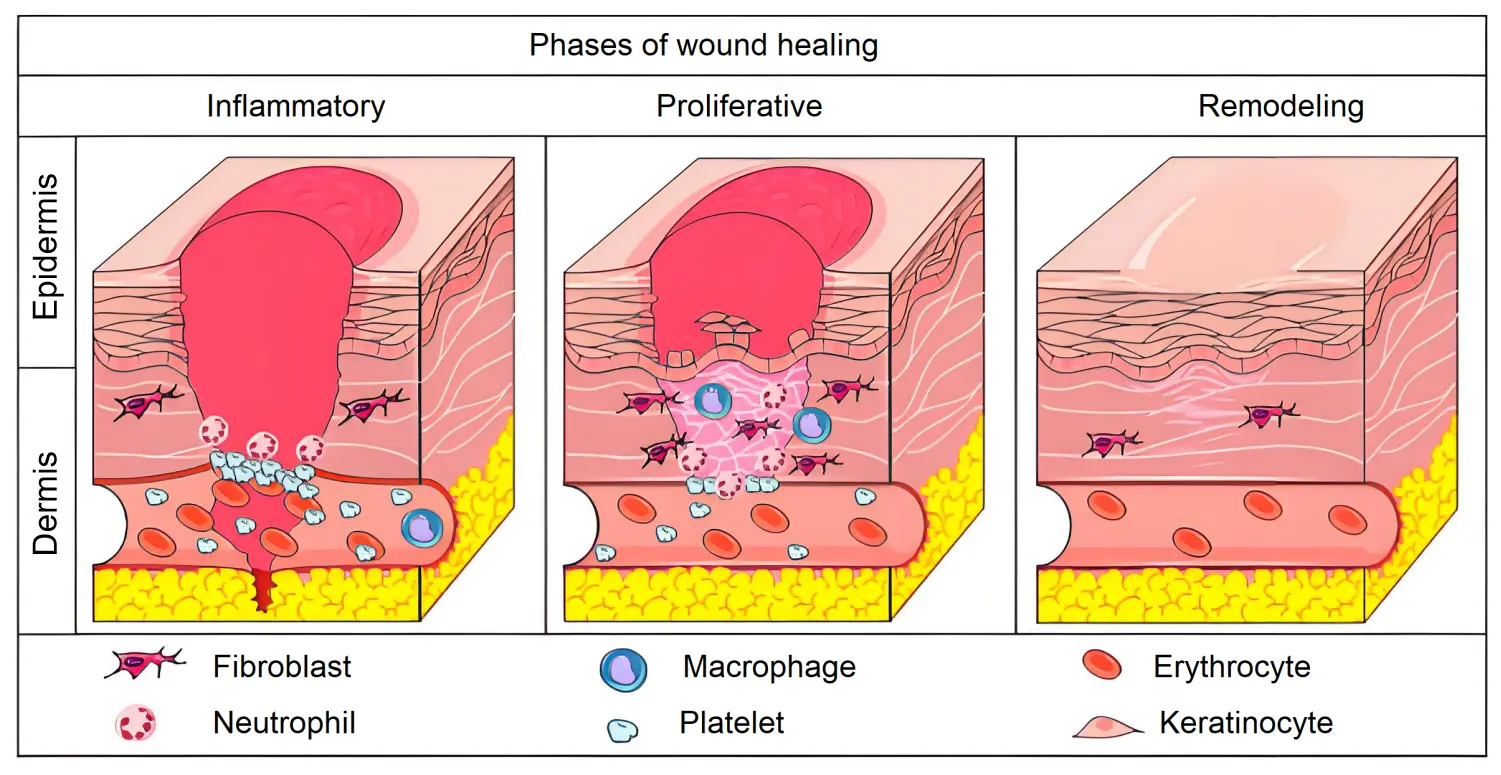
Figure 1. Phases of physiological wound healing. Inflammatory phase: there is the hemostasis of wounded area and acute inflammation through the release of cytokines, growth factors and the migration of leukocytes in the area. Proliferative phase: increase in the migration and proliferation of the keratinocytes, fibroblasts, endothelial cells and leukocytes in the wound. Increase in the synthesis of extracellular matrix components and improve of angiogenesis and re-epithelialization mechanisms. Remodeling phase: extracellular matrix remodeling, with substitution of collagen III for collagen I[8].
(i) Homeostasis: Following injury, the immune system rapidly initiates a response by activating various humoral and cellular signals. Key players such as antibodies, neutrophils, macrophages, and lymphocytes are mobilized to control pathogen invasion, which can impede the normal healing process. A crucial event during this phase is the formation of a platelet-fibrin clot that swiftly controls hemorrhage and halts bleeding. This clot serves as a physical barrier, protecting the body from excessive blood loss and regulating the hemostatic process[9,10].
(ii) Inflammation: This phase is characterized by localized swelling and redness resulting from the clearance of injured cells, growth factors and foreign elements, such as bacteria, from the wound site. Neutrophils and macrophages release inflammatory cytokines including interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ), which amplify the inflammatory response during the wound healing process[11].
(iii) Proliferation: As inflammation subsides and wound contraction begins, revascularization becomes necessary to restore oxygen supply to the wound bed. Fibroblasts and epithelial cells migrate to the wound site, promoting cellular proliferation and differentiation. This phase is characterized by the release of growth factors such as epidermal growth factor, hepatocyte growth factor, fibroblast growth factor, and keratinocyte growth factor. These factors facilitate tissue regeneration by replacing damaged tissue with new extracellular matrix, thereby supporting re-epithelialization[12,13].
(iv) Remodelling: Following the proliferation phase, collagen fibers undergo reorganization while epithelial cells continue to proliferate and differentiate, leading to epithelial repair. Concurrently, granulation tissue matures into scar tissue, which strengthens the wound and ultimately results in scar formation and wound closure. Fibroblasts remodel the underlying dermis over the course of several months[14].
3. DFU and Pathogenesis
DFUs represent a disruption of the normal wound healing process, resulting in conditions that require targeted treatment to promote effective healing[15]. The metabolic abnormalities associated with diabetes impair lesion repair, primarily due to reduced production of pro-angiogenic growth factors and chemokines. DFUs are commonly characterized by a prolonged inflammatory phase, with an abnormal elevation in metalloproteinase activity that accelerates degradation of the extracellular matrix. This imbalance hinders adequate re-epithelialization and remodeling. In some cases, excessive metalloproteinase activity may also trigger cellular senescence, further compromising the healing process.
Moreover, metalloproteinases interfere with the activity of growth factors, thereby hindering angiogenesis and impairing tissue oxygenation. This leads to persistent hypoxia at the wound site and delays wound closure. As a result, open wounds become increasingly vulnerable to pathogenic infections, a condition further aggravated by the reduced phagocytic activity associated with hyperglycaemia. The combined effects of these factors significantly worsen clinical outcomes for affected individuals[16]. An overview of diabetic wound healing and its pathological features is illustrated in Figure 2.
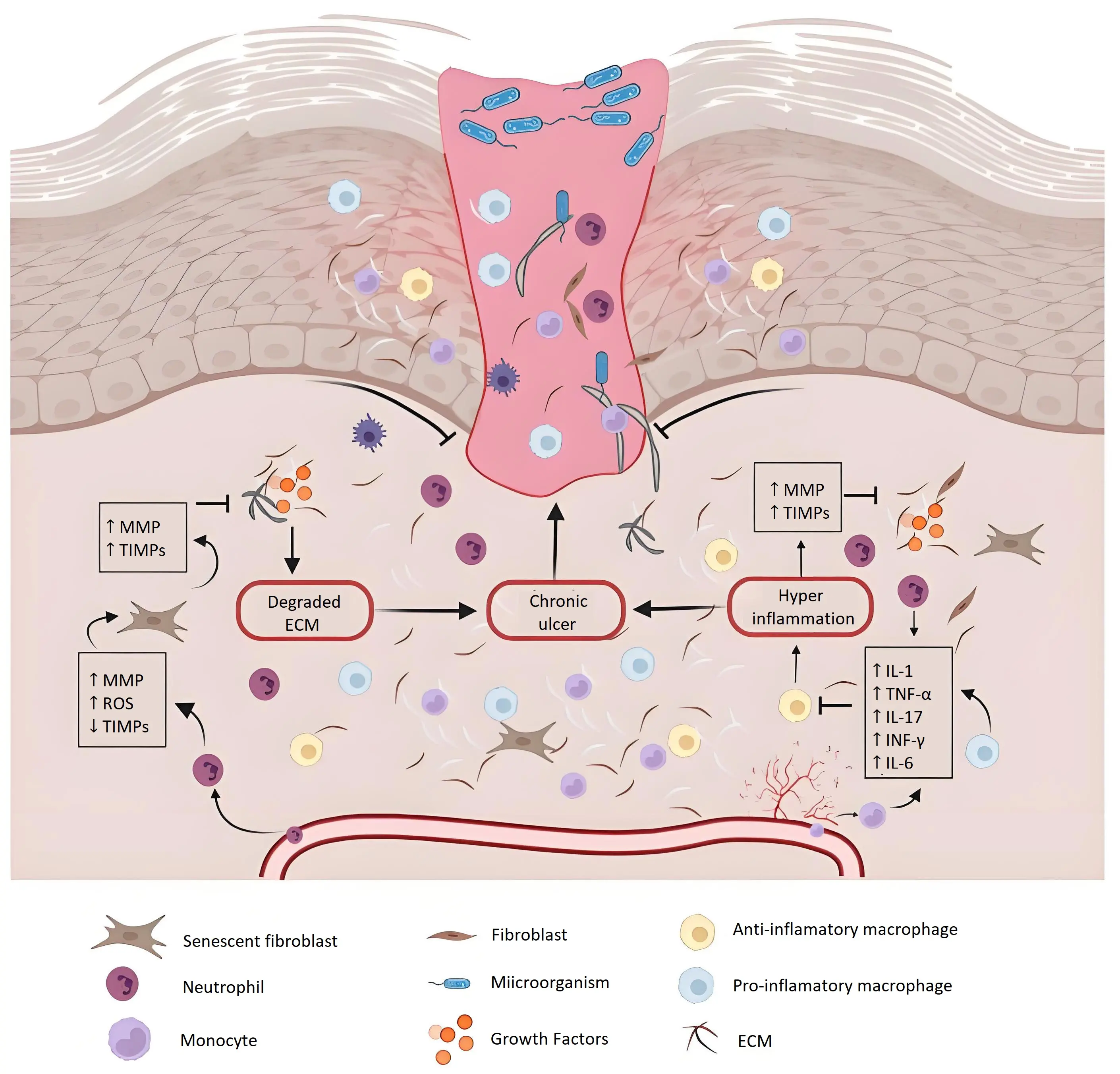
Figure 2. Image showing chronic wound healing. DFUs are characterized by continuous inflammation, persistent infections, and necrosis[19]. DFUs: diabetic foot ulcers; MMPs: Metalloproteinases; TIMPs: Tissue Inhibitors of Metalloproteinases; ROS: reactive oxygen species; ECM: extracellular matrix; TNF: tumor necrosis factor; IL: interleukins; IFN: interferon.
To address these challenges, tissue engineering strategies have enabled the development of hydrogel-based systems that function as supportive scaffolds, promoting appropriate cell interactions and facilitating the targeted delivery of bioactive agents to the wound site. These systems are designed to stimulate re-epithelialization, reduce inflammation, accelerate the healing process, and improve the quality of scar tissue formation[17-19].
3.1 Oxidative stress
Reactive oxygen species (ROS) and oxidative stress play critical roles in the pathogenesis of DFU. At physiological levels, ROS function as secondary messengers that support normal wound healing. However, under conditions of persistent hyperglycaemia in diabetes, excessive ROS production disrupts cellular homeostasis by activating inflammatory and oxidative damage pathways. This imbalance ultimately delays the wound healing process[20].
3.2 Nicotinic acid adenine dinucleotide phosphate (NADPH) depletion
Continuous hyperglycaemia causes the diversion of excess glucose into various metabolic or non-metabolic pathways. For example, it may be redirected into the polyol pathway, leading to increased activity of the sorbitol pathway. This, in turn, results in the depletion of the reducing agent NADPH, which is primarily produced by the pentose phosphate pathway. Such overconsumption of NADPH hinders the production of glutathione, an antioxidant that helps counteract oxidative stress. As a result, regular metabolism of proteins, lipids and DNA is impaired, contributing to mitochondrial injury and excessive generation of ROS[21].
3.3 Production of advanced glycation end products (AGEs)
Non-enzymatic glycation under hyperglycaemic conditions leads to the endogenous formation of AGEs. In addition, AGEs can be acquired exogenously through dietary intake. The accumulation of AGEs results in interactions with their receptor (RAGE), triggering various downstream signaling pathways that contribute to excessive oxidative stress, cellular damage, and dysregulated inflammatory immune responses. AGEs impair the functions of neutrophils and macrophages, particularly in terms of migration, adhesion, and phagocytosis. The signaling pathways activated by AGE–RAGE interactions include the mitogen-activated protein kinase pathway, the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, the Ras-mediated extracellular signal-regulated kinase (ERK1/2) pathway, and the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway[5].
3.4 Inhibition of Nrf2 signaling pathways
Nrf2 gene expression is important for the elimination of ROS[6]. Under normal physiological conditions, Nrf2 activity is regulated by the inhibitory molecule Keap1. Nrf2 undergoes ubiquitination and degradation, maintaining a concentration that balances oxidative reactions. When ROS levels are slightly elevated, compensatory activation of Nrf2 occurs through the weakening of the Nrf2/Keap1 interaction, resulting in an increased concentration of Nrf2. This promotes its translocation into the nucleus, where it binds with Maf proteins to form a complex. The complex then binds to antioxidant response elements, activating the transcription of various antioxidant genes, including glutathione, heme oxygenase-1, catalase, and quinone oxidoreductase 1.
However, under conditions of hyperglycaemia and diabetes, ROS levels exceed the capacity of this compensatory mechanism, leading to inhibition of the Nrf2 gene and a subsequent decrease in the expression of antioxidant molecules. This contributes to an imbalance in oxidative activity and exacerbates oxidative stress, resulting in abnormal inflammatory responses, cellular damage, and apoptosis[19]. Oxidative stress-related pathways thus play a critical role in cellular damage associated with DFU and contribute to delayed wound healing. Consequently, the use of natural biologics that target these pathways is regarded as a promising strategy to accelerate the healing process.
3.5 Neuropathies
Diabetic neuropathy is one of the primary causes of DFU. Several molecular pathways contribute to the development of neuropathy, including reduced Na+/K+-ATPase activity, the accumulation of AGEs, and the overproduction of ROS. In diabetes, the polyol pathway becomes overactive due to the high affinity of aldose reductase, leading to the accumulation of sorbitol. This accumulation impairs Na+/K+-ATPase activity, negatively affecting nerve conduction velocity and depleting cellular energy reserves. However, studies have shown that even aldose reductase-deficient diabetic mice develop neuropathy, suggesting that other hyperglycaemia-induced cellular processes also contribute to neuropathic damage. The accumulation of AGEs further exacerbates diabetic neuropathy through mechanisms such as altered nitric oxide synthase levels, impaired vasodilation, and reduced neural blood perfusion, ultimately leading to hypoxia and cell death[22].
Diabetic neuropathy may also result from reactive oxygen species generated due to the accumulation of AGEs or the diversion of glucose into the polyol pathway. This process leads to proton leakage back into the mitochondria without ATP production, causing mitochondrial damage through excessive ROS generation. Consequently, these events contribute to the loss of Schwann cells via demyelination, sensory deficits, axonal degeneration, and alterations in motor and autonomic nerves[20]. In addition to the molecular pathway disruptions described above, vascular and metabolic changes further contribute to the development of sensory, motor, and autonomic neuropathies.
Sensory neuropathy is characterized by a broad spectrum of symptoms, including paraesthesia, allodynia, hyperalgesia, sensory disturbances, and loss of protective sensations. The latter involves deficits in pain perception, temperature sensation, and proprioception[16-19]. The specific symptoms vary among diabetic patients depending on the types and properties of the affected nerve fibers[23]. Due to sensory loss, injuries often go unnoticed, resulting in delayed treatment. The repetitive nature of these traumas leads to cumulative micro-injuries that exacerbate the condition, ultimately causing the formation of calluses and ulcers, abnormal gait patterns, and increased morbidity.
Motor neuropathy manifests in various forms, including atrophy of the small muscles of the foot and glycosylation of tendons. Muscle wasting predominantly affects the extensor muscles, resulting in an imbalance between flexor and extensor muscles that leads to malpositioning of the toes[17,18]. Tendon glycosylation causes shortening and stiffness, which increases plantar pressure, particularly on the forefoot[17]. These biomechanical abnormalities contribute to foot deformities such as claw toes, hammer toes, equinus deformity, and an unsteady gait. Moreover, the resulting abnormal pressure distribution further elevates the risk of DFUs[24].
Autonomic neuropathy leads to impaired function of sebaceous and sweat glands, resulting in viscoelastic changes in the skin. This manifests as dry skin prone to spontaneous cracking and fissures, which subsequently become more susceptible to injuries, ulcers, and infections. These effects arise from a dysregulated neuroinflammatory response to noxious stimuli and microvascular damage, causing local edema and delayed healing. Subcutaneous haemorrhage is a complication associated with callus formation, stemming from the interplay of sensory, motor, and autonomic neuropathies. This process is exacerbated by repetitive minor traumas, leading to a range of complications, including gangrene and ultimately, foot amputation. Tissue ischemia, hypoxia, and hyperglycaemia in diabetic wounds impair normal wound healing, resulting in delayed recovery and increased risk of complications. It is estimated that approximately 19-34% of patients with diabetes develop DFUs. The pathophysiology of DFUs is multifactorial, primarily involving four main factors: peripheral arterial disease, peripheral neuropathy, cellular dysfunction, and infections[25-27].
3.6 Peripheral arterial disease
Peripheral arterial disease is a significant risk factor for the development of foot ulcers, affecting nearly 50% of patients with diabetic foot ulcers. Persistent hyperglycaemia induces endothelial dysfunction, characterized by reduced nitric oxide production, increased endothelin-1 activity, and elevated levels of antifibrinolytic and prothrombotic molecules, which collectively promote vasoconstriction and a hypercoagulable state. The hypermetabolic condition also leads to non-enzymatic glycosylation of proteins, resulting in thickening of the capillary basement membrane and decreased capillary elasticity, thereby causing hypoperfusion in the ulcerated regions[27]. Atherosclerosis is a well-recognized and severe complication of diabetes, associated with high mortality. Hyperglycaemia and dyslipidaemia are key contributors that accelerate its progression. Multiple pathways contribute to the pathogenesis of hyperglycaemia-induced atherosclerosis, with one major mechanism being the excessive production of ROS via the polyol pathway, protein kinase C activation, and AGE formation. These mediators disrupt hemostasis, leading to vasoconstriction and tissue hypoperfusion[28]. Furthermore, they activate inflammatory pathways, including the upregulation of transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1.
In the pro-inflammatory environment mediated by cytokines, macrophages are recruited to the site, where they phagocytose oxidized low-density lipoprotein (LDL), leading to the formation of foam cells that initiate the atherosclerotic process. This is followed by the migration of vascular smooth muscle cells to the arterial intima, resulting in the secretion and deposition of extracellular matrix components. Dyslipidaemia, frequently accompanying diabetes, accelerates this process by increasing circulating LDL levels. The progressive accumulation of lipids causes arterial stenosis, which reduces blood flow and leads to tissue hypoxia[29]. Atherosclerosis markedly elevates the risk of diabetic foot ulcer development, prolongs healing times, and increases the likelihood of ulcer recurrence. Peripheral arterial disease, characterized by severe ischemia and hypoxia, particularly in the lower limbs, is a common clinical manifestation of atherosclerosis. This ischemic milieu induces oxidative stress and stimulates the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6)[30].
Additionally, diabetes is associated with dysregulation of angiogenesis, as reflected by altered VEGF expression. Both overactivation and underexpression of the VEGF pathway have been observed in diabetes. Notably, diabetic retinopathy and neuropathy are linked to increased angiogenesis, whereas a significant reduction in angiogenesis occurs during the wound-healing process[31]. Levels of other growth factors, including fibroblast growth factor, epidermal growth factor, and platelet-derived growth factor, are also decreased in diabetes. In 1997, Beer et al. reported reduced PDGF expression in a diabetic animal model[9], and Qi et al. demonstrated that neutralization of PDGF in animal models improved wound healing[32]. Becaplermin, a recombinant human platelet-derived growth factor, remains the only FDA-approved topical pharmaceutical agent specifically indicated for the treatment of diabetic foot ulcers[31-33].
Impaired blood flow to the ulcerated foot, combined with hyperglycaemia and atherosclerosis, compromises the local immune response. Tissue hypoperfusion reduces the number of immune cells reaching the wound site, allowing infections to progress unchecked. Moreover, despite antibiotic administration, DFUs exhibit prolonged healing times due to the insufficient antibiotic concentration at the injury site[34]. Diabetic foot infections are often caused by a single pathogen, predominantly Staphylococcus aureus, with methicillin-resistant S. aureus (MRSA) accounting for 16.78 to 30% of reported cases[35]. Although MRSA infections do not appear to increase mortality, they are associated with higher hospitalization rates and an elevated risk of amputation[27]. Chronic infections are frequently polymicrobial, involving S. aureus, Streptococcus spp, coagulase-negative staphylococci, enterococci, and various Gram-negative bacteria[36]. Among fungal pathogens, Candida species play a significant role in the pathogenesis of fungal diabetic foot infections[26]. The primary treatment strategy for diabetic foot infections is antibiotic therapy, with amputation considered in severe or refractory cases[37].
4. Natural Polymer-Based Wound Dressing Materials for DFU
Natural polymers sourced from microbial, animal, or plant origins are primarily composed of proteins or polysaccharides that can closely mimic the cellular microenvironment and undergo controlled biodegradation. Despite their inherent advantages, these materials face several challenges, including batch to batch variability, high production costs particularly for protein-based polymers, potential risks of disease transmission, and limited mechanical stability. Such limitations constrain their widespread application. However, chemical modifications and blending with synthetic polymers present promising strategies to overcome these issues. Consequently, natural polymers remain a focal point of ongoing research for wound dressing applications especially in the treatment of DFU[38]. Figure 3 illustrates the chemical structures of several key natural polymers used in DFU wound dressings, while Table 1 summarizes the principal natural polymer based hydrogels employed in diabetic wound healing[19].
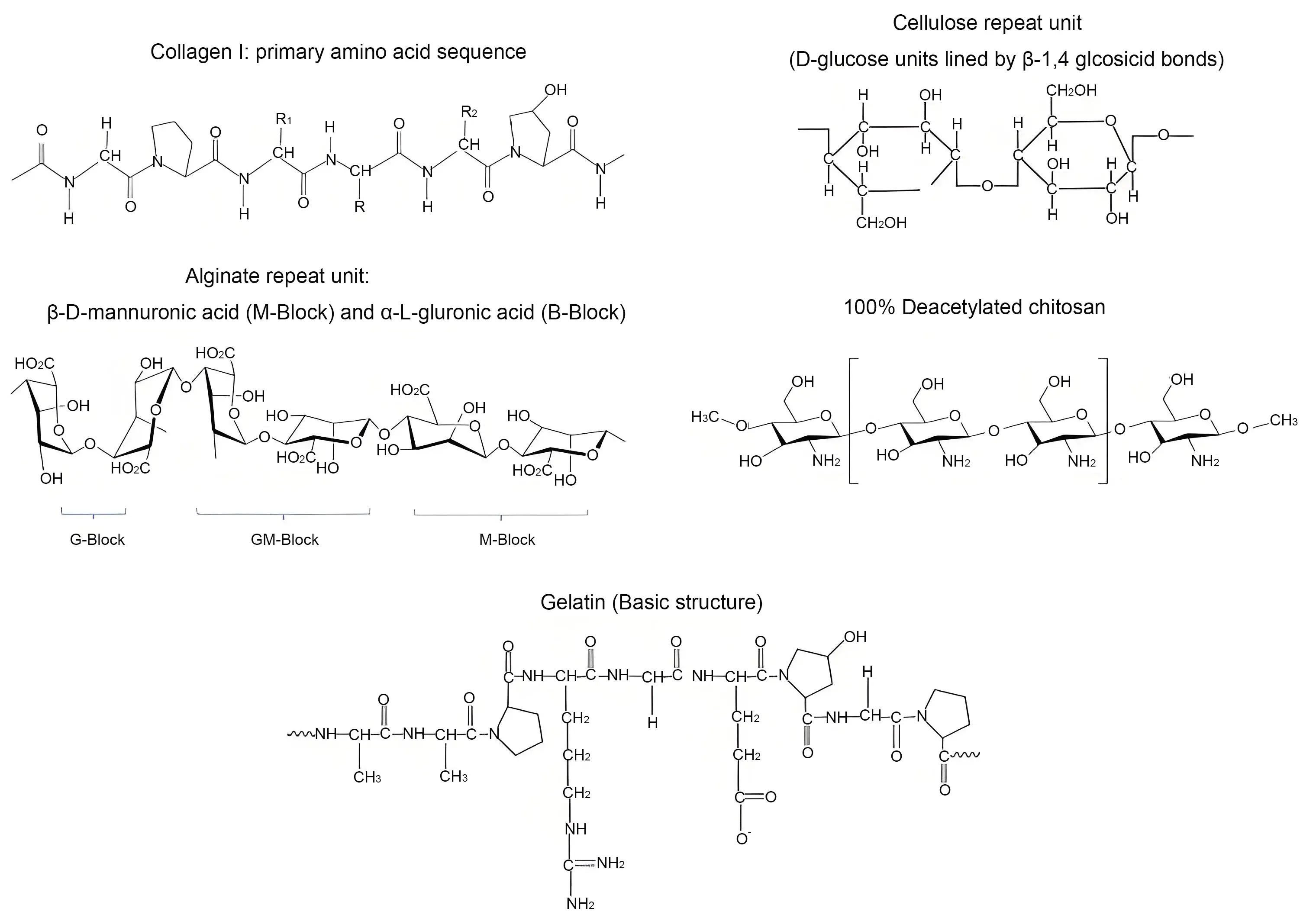
Figure 3. Chemical structures of a few important natural polymers used in wound dressing for DFU[19]. DFU: diabetic foot ulcer.
| Hydrogel material | Functional components | Properties |
| Sodium alginate | Deferoxamine and copper nanoparticles | Enhanced antimicrobial effect and angiogenesis, and reduced inflammatory response. |
| Sodium alginate and pectin | Simvastatin | Accelerated wound closure, fibroblast proliferation and collagen production. |
| Silk nanofiber | Deferoxamine | Enhanced collagen deposition and wound healing rate. |
| Gelatin and hyaluronic acid | Thrombomodulin | Enhanced granulation tissue formation, collagen deposition and angiogenesis. |
| Chitosan | D-(+) raffinose pentahydrate | Increased bacterial effect and accelerated wound healing. |
| Chitosan and starch | Chitosan and silver nanoparticles | Improved wound healing rate. |
| Collagen and sodium alginate | Curcumin loaded chitosan nanoparticles | Reduced inflammation, enhanced cell adhesion and accelerated wound healing. |
| Gelatin and hydroxyphenyl propionic acid | Interleukin or macrophage inflammatory protein | Increased cell infiltration, neo-vascularisation and collagen deposition. |
4.1 Chitin, chitosan and derivatives
Chitin is a biopolymer found in fungal cell walls that provides reinforcement and resilience through its strong fibers formed by weak inter-fiber links of 1-4 linked N-acetyl-2-amino-2-deoxy-D-glucose units[39]. Chitosan, derived from chitin, is a linear polysaccharide composed of D-glucosamine (deacetylated units) and N-acetyl-D-glucosamine (acetylated units) connected by β-(1→4) bonds. Its molecular weight ranges from 50 to 1,000 kDa, with degrees of deacetylation varying from 30 to 95%, depending on the source and processing methods. Chitosan exhibits strong antimicrobial properties, making it a promising candidate for wound dressings, especially in the treatment of DFU. It has shown great potential in wound healing applications. For instance, a multifunctional hydrogel composed of quaternized chitosan, tannic acid, and ferric iron has been developed for wound closure and healing. This hydrogel demonstrates excellent adhesion, antibacterial activity, antioxidant capacity, and biocompatibility, which collectively accelerate wound healing and tissue regeneration. Its unique properties including adhesion, self healing, conductivity, and photothermal effects make it a promising option for wound management and treatment of infected wounds, with significant improvements observed in wound closure rates and tissue regeneration[40].
4.2 Cellulose
Cellulose is a natural polymer consisting of linear chains made up of glucose units linked through oxygen atoms between glucose molecules[41]. While plant derived cellulose typically contains impurities like lignin and hemicellulose, leading to lower crystallinity, bacterial cellulose is purer and exhibits superior properties for biomedical applications[42]. Cellulose derivatives are particularly interesting for their wound management capabilities, including effective absorption and retention of wound exudates, which creates the optimal moist environment essential for chronic wound healing[43]. Cellulose containing growth factors promote wound healing through release of these factors which support fibroblast migration and proliferation. Additionally, cellulose has shown antimicrobial properties which further aid in wound recovery[38].
Bacterial cellulose is a valuable platform for nanoparticle loading, addressing challenges related to aggregation and ion release. For example, silver nanoparticles (AgNPs) incorporated into bacterial cellulose matrices release at low concentrations, minimizing cell toxicity while enhancing antibacterial activity. Eco-friendly bacterial cellulose/AgNPs composites developed using solar radiation and bacterial cellulose microfibers without the need for additional reducing agents have shown promise in improving wound healing outcomes[44].
Furthermore, a study comparing microbial cellulose dressings to Xeroform™ Petrolatum gauze in DFU demonstrated that microbial cellulose-treated ulcers healed in 32 days, compared to 48 days for the control group. The microbial cellulose dressings exhibited a 1.7-fold faster weekly wound closure rate, emphasizing the potential of microbial cellulose as an effective treatment for DFU[45].
4.3 Alginate
Alginate, a non-toxic and biodegradable polymer derived from brown algae and bacteria, has gained significant attention in wound healing due to its versatility and biocompatibility. The polymer is composed of α-l-guluronic acid (G) and β-d-mannuronic acid (M) residues linked via 1,4-glycosidic bonds. The properties of this material can be modified through chemical reactions[46,47]. High M-block content in alginate can induce greater cytokine production compared to G-blocks[48]. Calcium alginate, commonly used in wound dressings, undergoes ion exchange to absorb exudate, providing a moist healing environment, and is particularly effective for chronic, high exudate wounds. It absorbs bacteria and activates platelets, promoting healing and immune defence. However, in wounds with insufficient exudate, unconverted calcium alginate may form a crust that risks re-injury during dressing changes[49].
Alginate-based dressings have been enhanced with growth factors and drugs such as stromal-derived factor-1, phenytoin, ibuprofen or clindamycin to further improve DFU treatment. Commercial products like Medihoney and Algisite M highlight the effectiveness of alginate in DFU management. Additionally, a study evaluating a hydrogel enriched with sodium alginate and vitamins A and E showed reduced inflammatory infiltrate in the experimental group, suggesting potential benefits in DFU healing, though no significant difference in lesion area or PUSH sub scores was observed[50]. These findings underscore the broad potential of alginate dressings in wound healing and DFU management.
4.4 Fibrin
Fibrinogen, a protein synthesized in the liver, plays a key role in blood clot formation and wound healing. Upon activation by thrombin, fibrinogen is cleaved into fibrin, which polymerizes to form a network that stops bleeding. This fibrin network, created through end-to-middle domain associations and lateral fibril branching, is biocompatible and essential for hemostasis and tissue repair[51]. The interaction of fibrinogen with thrombin, fibroblasts, platelets, leukocytes, and endothelial cells are crucial in wound healing and regulating fibrinolysis[52,53]. The poor mechanical property of fibrin limits its use as a sole wound-healing material. This has been overcome through the development of fibrin nanocomposites. These nanocomposites offer promise for hemostasis, tissue repair, and infection defence[54]. Additionally, fibrin-based biomaterials including cross-linked fibrin composites are gaining attention in tissue engineering for drug and cell delivery[55].
Recent clinical studies have highlighted the potential of fibrin-based therapies in DFU treatment. A retrospective study at Tongji Hospital demonstrated that leukocyte- and platelet-rich fibrin (L-PRF) significantly improved DFU healing. After a median of three L-PRF doses, ulcer volume decreased substantially, with an 80% volume reduction achieved by week 7 and complete epithelialization in 11 patients by week 5[56]. Another study showed that autologous multilayered leukocyte, platelet, and fibrin (MLPF) patches improved DFU healing, with 79% of wounds healed by follow-up and a 72% reduction in average hospital stay, suggesting that MLPF patches are effective for treating hard-to-heal DFUs and reducing hospital readmissions[57]. These findings reinforce the therapeutic potential of fibrin-based biomaterials in the management of DFU.
4.5 Elastin
Elastin is a critical extracellular matrix protein that provides elasticity to tissues such as vasculature, skin, and lungs. It has a complex structure formed by crosslinked tropoelastin molecules. Elastin is also involved in cellular signaling, influencing processes such as chemotaxis, cell growth, and tissue homeostasis[58]. In the human body, elastin plays a pivotal role in modulating cellular behaviour. It supports fibroblast migration and proliferation, keratinocyte migration, and promotes angiogenesis in endothelial cells, all of which are vital processes in wound healing. However, in chronic wounds like venous leg ulcers and DFU, elastin levels are significantly reduced in the upper dermis. Additionally, inflammatory degradation of vascular elastin is notably elevated in patients with type 2 diabetes[59]. Despite limited reports on elastin-based dressings for DFU treatment, a recent study demonstrated that a protein gel containing elastin-like peptides and keratinocyte growth factor enhanced re-epithelialization and granulation tissue formation in diabetic mice wounds, suggesting that elastin-based polymers could be promising for improving DFU healing[60]. This study highlights the potential of elastin as a therapeutic biomaterial for wound care.
4.6 Silk fibroin
Silk fibroin, derived from silkworm silk, is known for its excellent biocompatibility, biodegradability, and adjustable mechanical properties, making it a promising material for wound healing[61]. Silk fibroin consists of a high molar mass chain (~390 kDa) and a medium molar mass chain (~26 kDa). These two macromolecular chains form a complex through disulfide linkages. A glycoprotein is also associated with this structure via non-covalent interactions[62]. Recent studies have highlighted silk fibroin-based materials as effective wound dressings, particularly for DFU. For instance, a study involving neurotensin/gelatine microspheres/silk fibroin (NT/GMs/SF) dressings in a rat model showed enhanced healing, including improved macroscopic wound closure, reduced scarring, and increased fibroblast accumulation and collagen deposition at the wound site. Additionally, hydrogels based on silk fibroin and glycyrrhizin acid containing silver nanoparticles demonstrated effectiveness in promoting tissue regeneration in DFU[63,64].
The properties of silk fibroin can be further enhanced through chemical modification. For example, silk fibroin membranes treated with a strong base such as sodium hydroxide exhibit increased surface roughness, which improves fibroblast adhesion and hydrophilicity. This treatment resulted in a 2.3-fold increase in cell attachment, highlighting the potential of chemically modified silk fibroin materials for advanced wound healing applications[65]. However, despite these improvements, silk fibroin has limitations including poor mechanical strength and lack of inherent antimicrobial properties, which restrict its use as a standalone material in wound healing. To overcome these drawbacks, silk fibroin is often combined with other polymers or antimicrobial agents such as vancomycin or antimicrobial peptides[66]. These modifications enhance the material’s performance and expand its applicability in wound care.
4.7 Collagen and gelatin
Collagen is a crucial structural protein present in various animal tissues, including skin, bones, tendons, and cartilage, constituting about 30% of the total protein in the human body. It plays a vital role in maintaining tissue strength and stability and contributes significantly to the integrity of the extracellular matrix and connective tissue[67]. Gelatin, derived from the partial denaturation of collagen, is widely used in packaging due to its low cost, biodegradability, and excellent oxygen barrier properties[68]. Both collagen and gelatin are biodegradable, surface-active, and biocompatible, making them highly valuable in wound healing applications. Collagen functions as a chemotactic agent, creating a conducive environment for healing, while gelatin serves as a hemostatic agent with additional anti-inflammatory and antimicrobial properties that support tissue regeneration. During the proliferative phase of wound healing, collagen facilitates tissue growth, fibroblast proliferation, and granulation tissue deposition, whereas gelatin aids in cell migration. In the maturation phase, collagen enhances skin repair and helps reduce scarring by delivering essential nutrients to the wound site[69-71].
Studies have highlighted the effectiveness of collagen and gelatin in treating DFU. Collagen treatment resulted in a higher wound closure rate (73.5% compared to 56.6% with physiological saline) and faster recovery, along with a significant reduction in wound size after 12 weeks of treatment[72]. Collagen matrix dressings for neuropathic DFU showed a notable decrease in wound size at 4 weeks (54.5% versus 38.8% with saline) and a higher healing rate at 20 weeks (60% versus 35.5%), indicating that collagen accelerates the healing process[73]. Additionally, a case study reported successful healing of a stagnant DFU using gelatin, supporting its potential as an effective alternative for managing hard-to-heal wounds[74].
5. Synthetic Polymers
Synthetic polymers, including polyvinyl alcohol (PVA), polyethylene glycol (PEG), polyacrylic acid, polylactic acid, and poly(lactic-co-glycolic acid), are widely employed in DFU treatment due to their exceptional tunability, mechanical properties, and biocompatibility. These polymers provide enhanced wound coverage compared to their natural counterparts. Their hydrophilic nature supports a moist wound environment, which is essential for cell proliferation and granulation tissue formation. This promotes faster healing and minimizes complications in chronic wounds[75,76]. The chemical structures of some important synthetic polymers used in wound dressings are shown in Figure 4. The synthetic and semi-synthetic hydrogels developed for diabetic wound healing are summarized in Table 2[19].
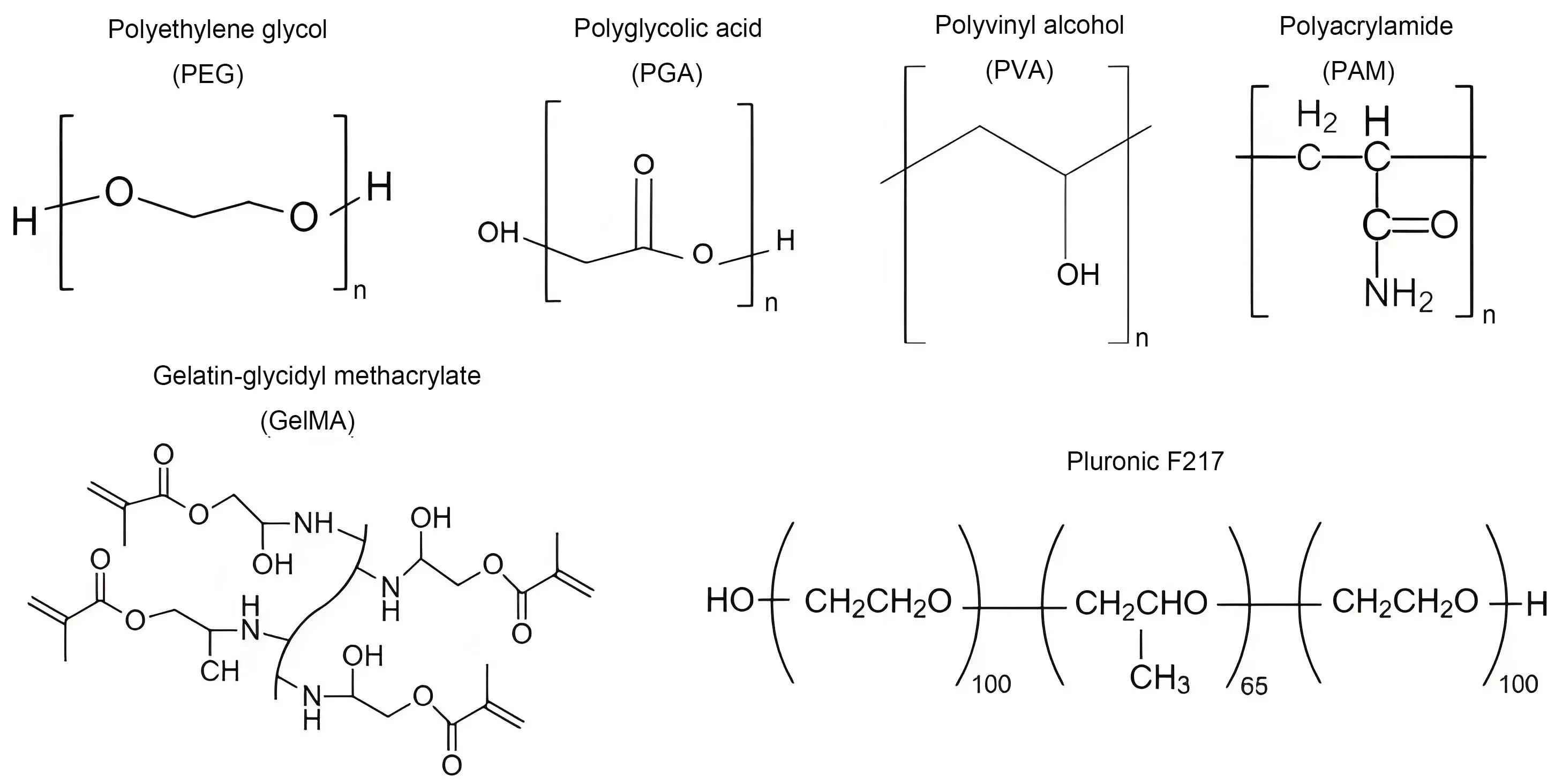
Figure 4. Chemical structures of synthetic polymers used for the design of hydrogel dressings for DFU treatment[19]. DFU: diabetic foot ulcer.
| Hydrogel material | Functional component | Properties |
| Gelatin methacrylate/PEGDA | Tazarotene, exosomes (derived from HUVEC) | Accelerated collagen deposition, epithelial cells regeneration, and angiogenesis. |
| Gelatin methacrylate and poly(L-lysine) | Vascular endothelial growth factor-mimetic peptide | Enhanced antibacterial effect, improved collagen deposition, angiogenesis and re-vascularization. |
| PLGA-PEG-PLGA | Copper-oxide, curcumin, and metformin hydrochloride | Enhanced cell migration, neovascularization, collagen formation, and reduction in oxidative stress. |
| Pluronic F-127 (20 %) | Ag nanoparticles with mesoporous silica containing gentamicin | Enhanced collagen production and faster wound healing of infected wounds. |
| Guanosine quadruplex | Hemin and GOX | Higher antibacterial effect and decreased glucose concentration in wound. |
| Chitosan and polyvinyl acetate | EGF-loaded nanoparticles, polyhexamethylene biguanide, and emulsion of perfluorocarbon | Higher anti-inflammatory effects, enhanced collagen production and wound closure. |
| Chitosan and polyvinyl acetate | Chitosan nanoparticles with human EGF and silver ions | Enhanced antibacterial effect, tissue maturation and wound closure. |
| Carboxy methyl chitosan and poly(dextran-g-4-formyl benzoic acid) | Peptide-modified nanofibers | Enhanced antibacterial and angiogenic effect. Reduced inflammatory response. |
| Quaternized chitosan and oxidised hyaluronic acid | Metal oxides containing α-lipoic acid | Increased collagen deposition, cell proliferation, neovascularization, and accelerated wound healing. |
| Polyacrylamide, gelatin and ε-polylysine | None | Increased formation of granulation tissue, collagen deposition and angiogenesis. |
| Hyperbranced PEG and thiolated hyaluronic acid | Encapsulated adipose-derived stem cells | Reduced inflammatory response, increased angiogenesis and re-epithelization. |
| PMMA and PVA | Chitosan, collagen and silver nanowires | Enhanced collagen production and reduced inflammatory response. |
| Phenylboronic acid modified chitosan, PVA, benzaldehyde capped PEG | Insulin and fibroblasts | Improved control of glucose levels in wounds, increase neovascularization, and enhanced wound closure rate. |
| Chitosan and PEG | Silver nanoparticles | Increased antibacterial effect, accelerated wound healing. |
| γ-polyglutamic acid | Human cell-free fat extract | Improved cell proliferation, collagen deposition, and angiogenesis. |
| Silk fibroin and polyvinyl pyrrolidone | Curcumin and L-carnosine | Enhanced wound healing. Significant antibacterial and anti-inflammatory effects. |
| Ferrocene, hyaluronic acid, rhein, and β-cyclodextrin. | None | Enhanced wound healing. |
| Chitosan and polyurethane | Bone marrow mononuclear cells (injected into edge of wound prior to application of hydrogel) | Hemostatic and anti-inflammatory effect, and enhanced wound healing. |
| 4-carboxybenzaldehyde, PEG, glycol chitosan, and silk fibroin | None | Enhance angiogenesis, re-epithelialization, nerve repair and wound healing rate. |
| N-carboxyethyl chitosan, adipic acid dihydrazide | Insulin | Significant reduction of glucose levels in wounds, decreased inflammation, and increased collagen production and re-epithelialization. |
| 4,5-imidazole dicarboxylic acid, zinc nitrate, deferoxamine mesylate and GOX | Deferoxamine mesylate | Enhanced antibacterial and angiogenesis. |
| PTFE and PU | Calcium alginate with Chlorella vulgaris and Bacillus licheniformis | Enhanced wound healing effect. |
| Cecropin-modified hyaluronic acid, oxidised dextran, and platelet-rich plasma | None | Accelerated wound healing and increased mature collagen content. |
PEGDA: polyethylene glycol diacrylate; HUVEC: human umbilical vein endothelia cells; GOX: glucose oxidase; EGF: epidermal growth factors; PMMA: poly(methylmethacrylate); PVA: polyvinyl alcohol; PTFE: Polytetrafluroethylene (Teflon); PU: Polyurethan.
5.1 Mechanical and structural advantages of synthetic polymers
Synthetic polymer-based hydrogels possess superior mechanical properties and high durability, which are crucial for DFU management. Hydrogels formed from these polymers, such as PEG or PLGA, exhibit high tensile strength and flexibility, allowing them to conform seamlessly to wound contours while maintaining structural integrity[77]. The hydrophilic nature of these materials ensures consistent moisture retention, preventing desiccation and supporting a stable, physiologically hydrated environment conducive to tissue regeneration[78].
Furthermore, the tunable degradation rates of synthetic polymers make them ideal for extended wound management, reducing the frequency of dressing changes. PLGA-based biodegradable hydrogels developed by Rahimzadeh et al. were effective in improving wound healing time and minimizing premature material degradation[79]. were effective in improving wound healing time and minimizing premature material degradation[80]. By balancing mechanical and biological properties, synthetic polymers offer promising solutions for addressing the unique challenges of DFU[81].
5.2 Drug delivery and infection control
Synthetic polymers have revolutionized drug delivery for DFU treatment by enabling the controlled and sustained release of therapeutic agents such as antibiotics, growth factors, and anti-inflammatory drugs. Hydrogels derived from polymers like PEG and PVA can encapsulate active agents and release them in a sustained manner, maintaining optimal concentrations at the wound site and reducing systemic side effects[82]. A notable advancement in this field is the incorporation of antimicrobial agents into synthetic polymer matrices to enhance infection control. For example, hydrogels embedded with silver nanoparticles have been shown to significantly reduce bacterial colonization and biofilm formation, which are critical challenges in DFU management[83]. Mahmood et al. demonstrated that synthetic hydrogels loaded with silver and iodine not only enhanced antibacterial activity but also disrupted biofilms, thereby facilitating effective wound closure[75].
Additionally, recent innovations in pH-sensitive and thermoresponsive hydrogels have introduced a new paradigm in DFU management. These so-called “smart” hydrogels adapt to the wound microenvironment, releasing therapeutic agents more effectively in response to local pH changes or temperature variations, thus increasing treatment efficacy[84]. Wang et al. explored the role of thermoresponsive hydrogels in targeted drug delivery and reported accelerated healing outcomes in DFU models[82]. Chitosan-based hydrogels incorporated with silver nanoparticles and ibuprofen exhibited improved anti-inflammatory properties and augmented granulation tissue formation[85,86]. Such advancements in synthetic polymer technology provide a robust platform for addressing challenges associated with infection and chronic inflammation in DFU.
5.3 Emerging innovations in synthetic polymer hydrogels
Nanocomposite hydrogels, which incorporate nanoparticles into synthetic polymer matrices, represent a significant milestone in DFU management. These hydrogels offer enhanced mechanical properties, improved oxygen permeability, and superior cell adhesion, which are crucial for promoting faster wound healing[87,88]. Wahid et al. demonstrated that synthetic hydrogels with embedded nanoparticles exhibit increased antibacterial activity and facilitate fibroblast proliferation, leading to reduced healing times[89].
Another breakthrough is the use of 3D printing to create hydrogel dressings that are customized to the unique shapes and depths of DFU wounds. Such materials ensure better fit and localized drug delivery[90]. Renuka et al. highlighted the advantages of 3D printed hydrogels with tailored mechanical properties for enhanced wound coverage and tissue repair[91]. Also, Jin et al.[92] integrated pH responsive, colour changing mesoporous resin beads into alginate fibers. Using 3D printing, they created hydrogel dressings equipped with porous pH sensor arrays (Figure 5). This novel method allows for real-time monitoring of pH levels within the hydrogel dressings, enabling targeted interventions to enhance wound healing. The developed hydrogel dressing not only provides immediate data on wound conditions, such as the extent of bacterial infection and the release of antibiotics, but also indicates these changes through colour variations.
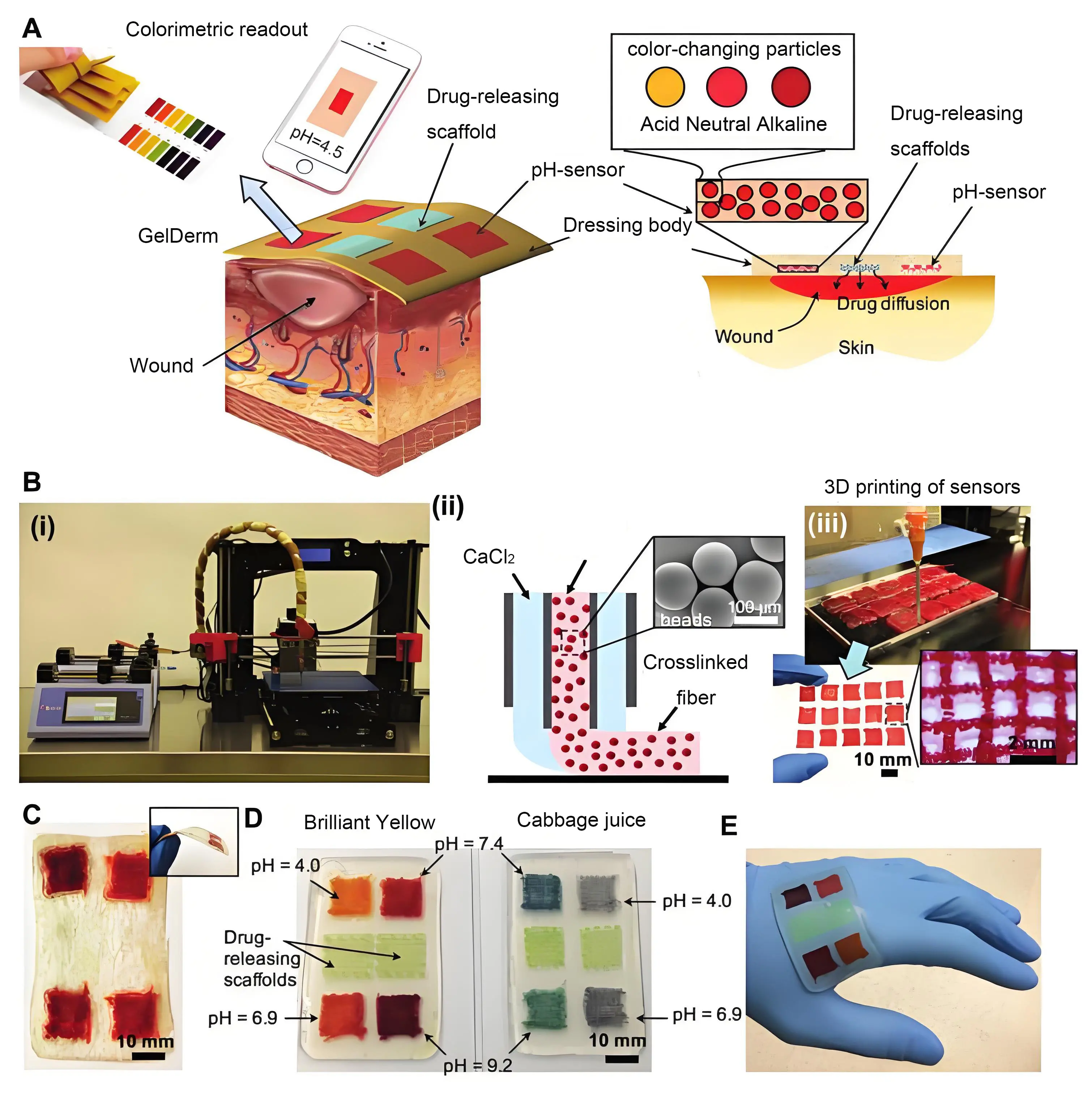
Figure 5. (A) Schematic representation of dressing treatment of epidermal wounds, with pH-sensitive and drug-eluting components; (B) (i) Porous sensors were fabricated using a 3D bioprinter equipped with a co-axial flow microfluidic nozzle; (ii) Schematic of fiber deposition using the co-axial flow system; (iii) 3D printer can be programmed to produce arrays of porous sensors for fabrication of large-scale dressings; (C) Dressings can be lyophilized and sterilized for storage and transportation; (D) Synthetic Brilliant Yellow and naturally derived cabbage juice were used as model pH indicators for the fabrication of the sensors. Sensor arrays enable detecting spatial variations of pH on the wound site. Drug-eluting scaffolds release high doses of antibiotics at the wound site to eradicate the bacteria that may remain on the wound site each time the dressing is replaced; (E) The multi-purpose dressing can maintain a conformal contact with irregular surfaces[92].
Stem cell-laden hydrogels, formulated using synthetic polymer bioinks, represent another emerging strategy. Astaneh and Fereydouni reported that these hydrogels accelerated healing in preclinical DFU models by combining the therapeutic benefits of stem cells with the structural support of hydrogels[93,94]. Such innovations reflect a broader shift toward personalized medicine, with synthetic polymers offering a robust platform for next-generation DFU therapies[76]. However, synthetic polymers have certain limitations, including cytotoxicity, low biocompatibility, and the potential to disrupt cellular environments. These issues restrict their clinical translation. Sanjarnia et al. highlighted that polymeric materials can interfere with cellular function, raising concerns regarding toxicity and immune responses[95]. Overcoming key challenges related to biocompatibility and regulatory compliance may enable the clinical application of these materials in DFU management[96-98].
6. Stem Cell Loaded Hydrogels
Mesenchymal stem cells, which originate from the mesoderm, are a type of adult stem cell found in the bone marrow, adipose tissue, umbilical cord, and placenta[99]. They possess typical stem cell characteristics such as self-renewal and multi-lineage differentiation. In addition to these properties, mesenchymal stem cells can regulate angiogenesis, cell recruitment, tissue remodelling, and immune responses, highlighting their role in tissue repair. One of the most common complications of diabetes is the development of DFU, which are often recurrent and prone to infection. Hydrogels loaded with adipose tissue- and bone marrow-derived stem cells have been shown to promote angiogenesis at the wound site. These stem cell-laden hydrogels can mobilize and establish a supportive microenvironment for ischemic tissue, thereby enhancing neovascularization and accelerating wound healing. Mesenchymal stem cells also initiate anti-inflammatory responses by modulating the immune system, which increases collagen deposition and promotes wound repair. Additionally, hydrogels containing topical embryonic stem cells have demonstrated positive effects on wound healing in diabetic mice by increasing the proliferation of Ki-67-positive cells and elevating regulatory T-cell levels[100-104].
7. Smart Hydrogels and Their Role in Personalized Care
Diabetes-related wounds exhibit several distinctive characteristics, including elevated blood glucose levels, persistent inflammation, fluctuating pH, insufficient oxygen supply, impaired angiogenesis, and fragile granulation tissue. These factors collectively delay the healing of DFU. To address these challenges, personalized treatment approaches have become increasingly important. Smart or intelligent hydrogels that respond to external stimuli such as pH, temperature, ionic strength, and biomolecules have been explored for DFU treatment and personalized care. These stimuli-responsive hydrogels enable continuous monitoring of wound healing stages, allowing for tailored therapeutic interventions. Integrating monitoring and treatment functions into a hydrogel dressing holds great potential for significantly improving the treatment and management of DFU[105].
7.1 Glucose responsive hydrogels
High blood sugar in diabetic wounds causes changes in the surrounding cellular environment, triggering a series of complex biochemical reactions that hinder the healing process. These reactions include a significant reduction in macrophages, accumulation of advanced glycation end products, and release of ROS. Elevated blood glucose levels also lead to higher glucose concentrations around the injured site, promoting bacterial growth[106]. Zhao et al. developed a pH- and glucose-responsive hydrogel based on Schiff base and phenylboronate for the delivery of insulin and fibroblasts[107]. Changes in pH, caused by fluctuations in glucose concentration, hydrolyze the hydrogel matrix, resulting in faster insulin release. Zhu et al. created a glucose-responsive hydrogel with self-healing and adhesive properties by using dopamine-modified gelatin and phenylboronic acid-modified hyaluronic acid[108]. This gel was loaded with metformin and copper-coated dopamine nanoparticles to mimic the extracellular matrix. Drug release was activated in response to changes in pH and glucose concentration within the wound.
High blood sugar also impairs the normal healing process by reducing the amount of oxygen and nutrients delivered to the cells. Glucose oxidase has been utilized to decrease blood glucose levels near the wound site. Zhu et al. developed a flexible zwitterionic hydrogel capable of simultaneously measuring pH and glucose levels. This hydrogel was loaded with glucose oxidase, horseradish peroxidase, and a pH-sensitive dye (phenol red). Using this multifunctional dressing, blood glucose levels ranging from 0.10 to 0.10 and 10 × 10-3 M and pH fluctuations within a healing relevant range, typically between pH 5 and 7, were accurately monitored and detected. Such multifunctional dressings hold great potential to transform the treatment and management of DFU[107-109].
7.2 pH responsive hydrogels
The pH of healthy skin typically ranges from 4.8 to 5.7, but this can change when the skin is damaged and a wound forms. The pH becomes more acidic during the debridement and healing phases, then shifts toward alkaline near the completion of healing[110,111]. Certain physiological processes can also influence pH; for example, glycolysis increases lactic acid and carbon dioxide concentrations. Li et al. developed pH-responsive multifunctional hydrogels that monitor wound infection by detecting pH changes, thereby offering improved solutions for wound care[111]. Their hydrogel, based on modified hyaluronic acid and poly(6-aminohexanoic acid) containing strontium and dopamine, effectively regulated wound pH, promoted macrophage activity, and reduced inflammation, facilitating faster healing[112,113]. By modulating the wound’s pH, the hydrogel ensures adequate nutrient supply for fibroblast proliferation, supporting continuous healing and complete wound closure.
Regulated drug release at the wound site has been investigated using hydrogel dressings incorporating silver nanoparticles. Hu et al. developed a double-crosslinked pH-responsive hydrogel containing 1.2% silver nanoparticles along with antibiotics[114]. This hydrogel enabled sustained release of silver nanoparticles at the wound site for up to two weeks. Silver nanoparticles are well known for their antimicrobial properties, and the hydrogel dressing combining silver nanoparticles and antibiotics exhibited both antibacterial and angiogenic effects. Treatment of diabetic wounds infected with Staphylococcus aureus using this hydrogel dressing in rats resulted in full recovery within 10 days, compared to 17 days in the control group[115].
7.3 Anti-bacterial hydrogels
Smart hydrogels that respond to biomolecules and bacteria have been investigated for the treatment of DFU. These materials trigger the release of antimicrobial agents upon detecting the presence of bacteria[116]. Anti-bacterial hydrogels based on chitosan, loaded with pravastatin, demonstrated efficacy against Escherichia coli and MRSA. Zhang et al. studied the therapeutic mechanism of hydrogels containing protamine nanoparticles and hyaluronan oligosaccharides, revealing nanoparticle targeting of bacterial cell walls and the promotion of angiogenesis through endothelial cell formation and migration[117]. Composite hydrogels with enhanced antibacterial properties for treating severe infections have attracted considerable research attention in recent years. Novel antibacterial hydrogels have been developed to achieve synergistic activity against multiple bacterial species[118].
Polymersome hydrogel composites containing antibiotics, developed by Hong et al., exhibited long-lasting intrinsic antibacterial properties. In this system, polymersomes loaded with the antibiotic penicillin were grafted into the hydrogel network. Yang et al. developed a hybrid polydopamine/polyacrylamide hydrogel incorporated with poly(diallyl dimethyl ammonium chloride) brushes grafted from bacterial cellulose nanofibers[119]. This hydrogel demonstrated enhanced broad-spectrum antibacterial activity with low cytotoxicity. The positively charged poly(diallyl dimethyl ammonium chloride) brushes also promoted the growth and proliferation of negatively charged epidermal cells[120] (Figure 6).
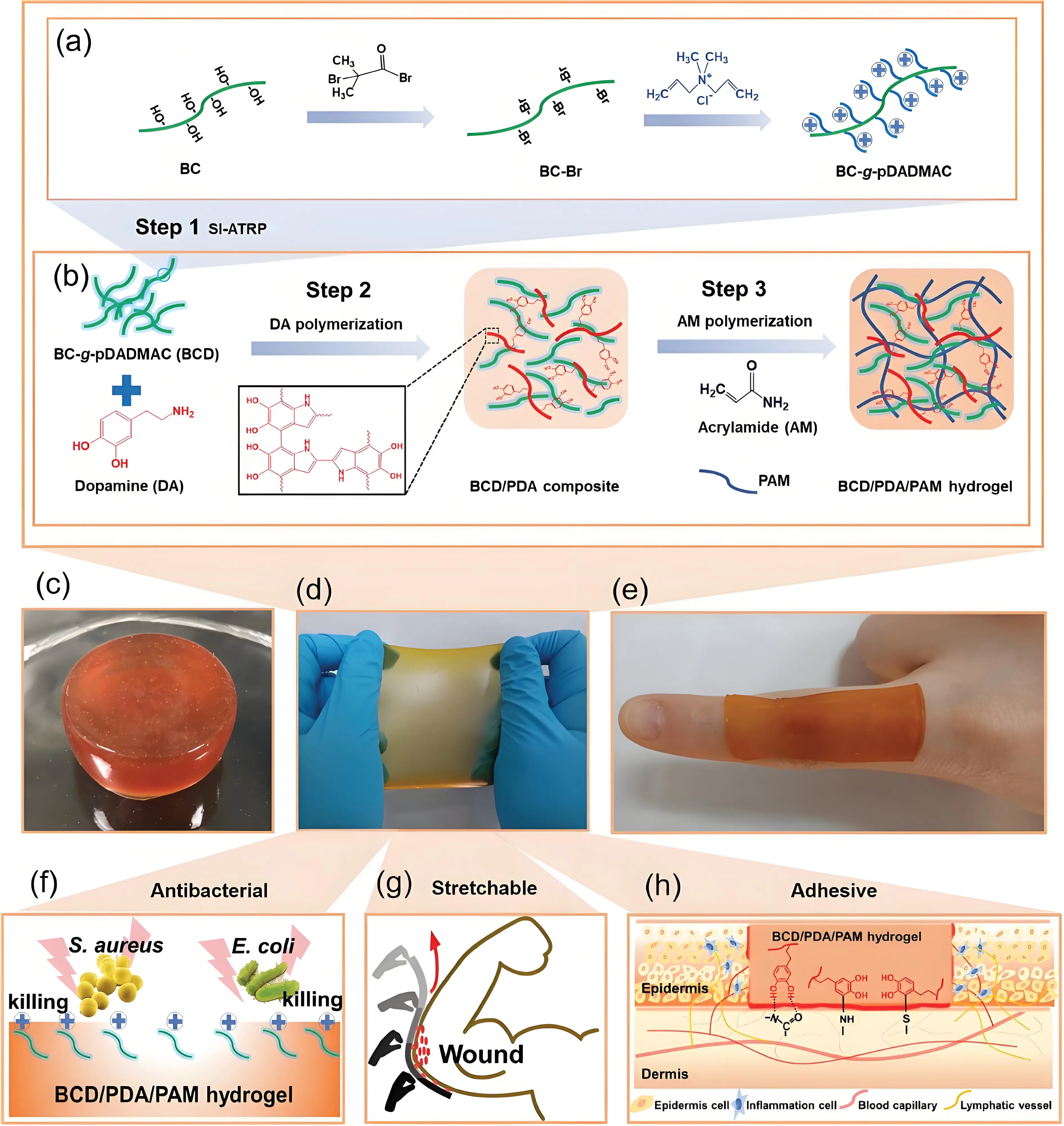
Figure 6. (a) Synthesis of polymersome based antibacterial hydrogel; (b) Formation of hydrogel; (c-e) optical images of the hydrogel; (f-h) Schematic illustration of antibacterial, stretchability and adhesiveness of the hydrogel[120]. BC: bacterial cellulose; BCD: BC-g-pDADMAC; PDA: polydopamine; PAM: polyacrylamide; pDADMAC: poly(diallyl dimethyl ammonium chloride).
In recent years, enzyme-based wound dressings have attracted considerable attention for the treatment of DFU. Enzymes and enzyme-like materials (analogues) can catalyze endogenous hydrogen peroxide at the wound site, generating oxygen that accelerates wound healing. Nanozymes, or nanoenzymes, are enzyme-based therapies that catalyze hydrogen peroxide into highly reactive and toxic hydroxyl radicals. These reactive free radical species disrupt bacterial cell membranes, leading to bacterial cell death in wounds. Various types of nanoenzymes used in wound care are summarized in Table 3[118].
| Nanozyme | Hydrogel | Properties |
| MnO2 nanosheets | PEGMA, glycidyl methacrylate and acrylamide | Broad-spectrum antibacterial activity, ROS-scavenging, O2 generation, accelerated wound healing |
| CeO2 nanorods | Dextran oxide and ε-polylysine | Broad-spectrum antibacterial activity, ROS-scavenging. |
| Polydopamine MnO2 | Polydopamine and thioctic acid | Broad-spectrum antibacterial activity, Oxygen generation by degradation of hydrogen peroxide, suppress inflammation. |
PEGDA: polyethylene glycol diacrylate; ROS: reactive oxygen species.
8. Conclusions, Limitations and Future Perspectives
Diabetes mellitus has a high prevalence among both young and elderly populations and poses a significant clinical challenge as well as a substantial financial burden on healthcare systems. One of the most severe complications is DFUs, which account for approximately 85% of diabetes-related amputations. The treatment of chronic DFUs is more complex and delayed compared to non-diabetic wounds. Both conventional and advanced wound dressings play a crucial role in DFU management by inhibiting microbial invasion and promoting the healing process. Despite progress, the development of an effective single clinical treatment for DFU remains a significant challenge. Nevertheless, new materials have been developed to address the difficulties associated with DFU treatment.
Due to their inherent properties, both physical and chemical hydrogels, as well as hydrogel-based materials, have been extensively explored as wound dressings for DFU. The swollen hydrogel networks closely mimic the characteristics of natural tissues and provide a moist environment at the wound bed, which facilitates wound repair. Smart hydrogels loaded with antibiotics, antioxidants, stem cells, growth factors, and insulin have been developed to accelerate DFU healing. Despite these advances in material technologies, hydrogel-based wound dressings still face several limitations in clinical translation. These challenges include poor mechanical strength, cytocompatibility issues, material degradation, and hurdles in commercialization. Mechanical strength can be enhanced through strategies such as double-crosslinking and the formation of interpenetrating polymer networks. Cytocompatibility and material erosion can be improved by selecting biocompatible and non-cytotoxic chemical crosslinkers or monomers. Scaling up production and commercializing multifunctional hydrogels with versatile properties remain significant obstacles. These challenges may be addressed through strong academic-industrial partnerships, which can facilitate clinical translation and the commercialization of novel hydrogel-based dressings for effective DFU treatment and management.
Authors contributions
Almarzooq G: Conceived the idea for the article, revised the manuscript, drafted the manuscript, conducted the literature search.
Deen GR: Conceived the idea for the article, revised the manuscript.
Alaysereen A, Salman Z: Drafted the manuscript, conducted the literature search.
Abdulrasool Z, Jaragh N, Hasan S, Tarig O: Conducted the literature search.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
None
Copyright
© The Author(s) 2025.
References
-
1. Zhao H, Wu Y, Xie Y, Li Y, Chen C, Li C, et al. Hydrogel dressings for diabetic foot ulcer: A systematic review and meta-analysis. Diabetes Obes Metab. 2024;26:2305-2317.
[DOI] -
2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843.
[DOI] -
3. Cao L, Adedjouma NG, Xu C, Shen KW, Laki F, Chen JY, et al. The role of post-mastectomy radiotherapy in elderly patients with 1-3 positive lymph nodes breast cancer: an international retrospective double-center study. Breast J. 2019;25:107-111.
[DOI] -
4. Park KH, Kwon JB, Park JH, Shin JC, Han SH, Lee JW. Collagen dressing in the treatment of diabetic foot ulcer: a prospective, randomized, placebo-controlled, single-center study. Diabetes Res Clin Pract. 2019;156:107861.
[DOI] -
5. Fard AS, Esmaelzadeh M, Larijani B. Assessment and treatment of diabetic foot ulcer. Int J Clin Pract. 2007;61:1931-1938.
[DOI] -
6. Morton LM, Phillips TJ. Wound healing update. Semin Cutan Med Surg. 2012;31:33-37.
[DOI] -
7. Dumville JC, O’Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013;7:CD009101.
[DOI] -
8. Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: An update from physiopathology to current therapies. Life. 2021;11(7):665.
[DOI] -
9. Goldberg SR, Diegelmann RF. Wound healing primer. Surg Clin N Am. 2010;90:1133-1146.
[DOI] -
10. Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol Life Sci. 2016;73:3861-3885.
[DOI] -
11. Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Mol Biol Cell. 2012;23(6):1068-1079.
[DOI] -
12. Caley MP, Martins VL, O’Toole EA. Metalloproteinases and wound healing. Adv Wound Care. 2015;4:225-234.
[DOI] -
13. Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG, Cid-Baez MA, Zamudio-Osuna MDJ, Martinez-Blanco MR, et al. Current therapeutic strategies in diabetic foot ulcers. Medicina. 2019;55:714.
[DOI] -
14. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229.
[DOI] -
15. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370-378.
[DOI] -
16. Straccia MC, D’Ayala GG, Romano I, Oliva A, Laurienzo P. Alginate hydrogels coated with chitosan for wound dressing. Mar Drugs. 2015;13:2890-2908.
[DOI] -
17. Koehler J, Verheyen L, Hedtrich S, Brandl FP, Goepferich AM. Alkaline poly(ethylene glycol)-based hydrogels for a potential use as bioactive wound dressings. J Biomed Mater Res A. 2017;105:3360-3368.
[DOI] -
18. Zhu J, Cheng H, Zhang Z, Chen K, Zhang Q, Zhang C, et al. Antibacterial hydrogels for wound dressing applications: Current status, progress, challenges, and trends. Gels. 2024;10(8):495.
[DOI] -
19. Güiza-Argüello VR, Solarte-David VA, Pinzón-Mora AV, Ávila-Quiroga JE, Becerra-Bayona SM. Current advances in the development of hydrogel-based wound dressings for diabetic foot ulcer treatment. Polymers. 2022;14(14):2764.
[DOI] -
20. Pang L, Lian X, Liu H, Zhang Y, Li Q, Cai Y, et al. Understanding diabetic neuropathy: focus on oxidative stress. Oxid Med Cell Longev. 2020;2020(1):9524635.
[DOI] -
21. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1-14.
[DOI] -
22. Marshall A, Alam U, Themistocleous A, Calcutt N, Marshall A. Novel and emerging electrophysiological biomarkers of diabetic neuropathy and painful diabetic neuropathy. Clin Ther. 2021;43(9):1441-1456.
[DOI] -
23. Mariadoss AVA, Sivakumar AS, Lee CH, Kim SJ. Diabetes mellitus and diabetic foot ulcer: Etiology, biochemical and molecular based treatment strategies via gene and nanotherapy. Biomed Pharmacother. 2022;151:113134.
[DOI] -
24. Wang K, Wang Y, Shi W, Shen K, Tao K, Ling R, et al. Diagnosis and treatment of diabetic foot ulcer complicated with lower extremity vasculopathy: Consensus recommendation from the Chinese Medical Association (CMA), Chinese Medical Doctor Association (CMDA). Diabetes Metab Res Rev. 2024;40(3):e3776.
[DOI] -
25. Deng H, Li B, Shen Q, Zhang C, Kuang L, Chen R, et al. Mechanisms of diabetic foot ulceration: A review. J Diabetes. 2023;15(4):299-312.
[DOI] -
26. Kim J. The pathophysiology of diabetic foot: a narrative review. J Yeungnam Med Sci. 2023;40(4):328-334.
[DOI] -
27. Song J, Liu A, Liu B, Huang W, Jiang Z, Bai X, et al. Natural biologics accelerate healing of diabetic foot ulcers by regulating oxidative stress. Front Biosci. 2022;27(10):285.
[DOI] -
28. Blagov AV, Markin AM, Bogatyreva AI, Tolstik TV, Sukhorukov VN, Orekhov AN. The role of macrophages in the pathogenesis of atherosclerosis. Cells. 2023;12(4):522.
[DOI] -
29. Afonso AC, Oliveira D, Saavedra MJ, Borges A, Simões M. Biofilms in diabetic foot ulcers: Impact, risk factors and control strategies. Int J Mol Sci. 2021;22(15):8278.
[DOI] -
30. Lyttle BD, Vaughn AE, Bardill JR, Apte A, Gallagher LT, Zgheib C, et al. Effects of microRNAs on angiogenesis in diabetic wounds. Front Med. 2023;10:1140979.
[DOI] -
31. Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol. 1997;109(2):132-138.
[DOI] -
32. Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y, et al. High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes. 2014;64(4):1407-1419.
[DOI] -
33. Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, et al. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics. 2019;8(4):193.
[DOI] -
34. Rubitschung K, Sherwood A, Crisologo AP, Bhavan K, Haley RW, Wukich DK, et al. Pathophysiology and molecular imaging of diabetic foot infections. Int J Mol Sci. 2021;22(21):11552.
[DOI] -
35. MacDonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21:770.
[DOI] -
36. Hawkins BK, Barnard M, Barber KE, Stover KR, Cretella DA, Wingler MJB, et al. Diabetic foot infections: A microbiologic review. The Foot. 2022;51:101877.
[DOI] -
37. Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013;9(7):7093-7114.
[DOI] -
38. Guo S, Yao M, Zhang D, He Y, Chang R, Ren Y, Guan F. One-step synthesis of multifunctional chitosan hydrogel for full-thickness wound closure and healing. Adv Healthc Mater. 2022;11(4):2101808.
[DOI] -
39. Jafernik K, Ladniak A, Blicharska E, Czarnek K, Ekiert H, Wiacek AE, Szopa A. Chitosan-based nanoparticles as effective drug delivery systems—A review. Molecules. 2023;28(4):1963.
[DOI] -
40. Collard FX, Blin J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev. 2014;38:594-608.
[DOI] -
41. Portela R, Leal CR, Almeida PL, Sobral RG. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;12(4):586-610.
[DOI] -
42. Pita-Vilar M, Concheiro A, Alvarez-Lorenzo C, Diaz-Gomez L. Recent advances in 3D printed cellulose-based wound dressings: A review on in vitro and in vivo achievements. Carbohydr Polym. 2023;315:121298.
[DOI] -
43. Hou S, Xia Z, Pan J, Wang N, Gao H, Ren J, Xia X. Bacterial cellulose applied in wound dressing materials: Production and functional modification—A review. Macromol Biosci. 2024;24(2):2300333.
[DOI] -
44. Solway DR, Clark WA, Levinson DJ. A parallel open-label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. Int Wound J. 2011;8:69-73.
[DOI] -
45. Paques JP, van der Linden E, van Rijn CJ, Sagis LM. Preparation methods of alginate nanoparticles. Adv Colloid Interface Sci. 2014;209:163-171.
[DOI] -
46. Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37(1):106-126.
[DOI] -
47. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42.
[DOI] -
48. Peng W, Li D, Dai K, Wang Y, Song P, Li H, et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int J Biol Macromol. 2022;208:400-408.
[DOI] -
49. Barbosa MG, Carvalho VF, Paggiaro AO. Hydrogel enriched with sodium alginate and vitamins A and E for diabetic foot ulcer: a randomized controlled trial. Wounds. 2022;34(9):229-235.
[DOI] -
50. National Cancer Institute. Fibrinogen. NCI Dictionary of Cancer Terms. Available from: https://chatgpt.com/c/6833b98e-43a8-800a-ab44-a683d917dbc1
-
51. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894-1904.
[DOI] -
52. Weisel JW, Litvinov RI. Fibrin formation, structure and properties. In: Parry D, Squire J, editors. Fibrous Proteins: Structures and Mechanisms. Cham: Springer; 2017. p. 405-456.
[DOI] -
53. Bayer IS. Advances in fibrin-based materials in wound repair: A review. Molecules. 2022;27(14):4504.
[DOI] -
54. Otero AIP, Fernandes JCH, Borges T, Nassani L, Castilho RM, Fernandes GVO. Sinus lift associated with leucocyte-platelet-rich fibrin (second generation) for bone gain: A systematic review. J Clin Med. 2022;11(7):1888.
[DOI] -
55. Wang F, Zhang XL, Zhang J, Gong S, Tao J, Xiang H, et al. Therapeutic effectiveness of leukocyte-and platelet-rich fibrin for diabetic foot ulcers: A retrospective study. Curr Med Sci. 2024;44(3):568-577.
[DOI] -
56. Mendivil JM, Henderson LC, Olivas OS, Deanda MA, Johnson ML. Retrospective data analysis of the use of an autologous multilayered leukocyte, platelet, and fibrin patch for diabetic foot ulcers treatment in daily clinical practice. Adv Skin Wound Care. 2023;36(11):579-585.
[DOI] -
57. Silva SS, Fernandes EM, Pina S, Silva-Correia J, Vieira S, Oliveira JM, et al. Polymers of biological origin. Compr Biomater II. 2017;2:228-252.
[DOI] -
58. Yan L, Wang Y, Feng J, Ni Y, Zhang T, Cao Y, et al. Mechanism and application of fibrous proteins in diabetic wound healing: A literature review. Front Endocrinol. 2024;15:1430543.
[DOI] -
59. Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, et al. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc Natl Acad Sci USA. 2011;108(3):1034-1039.
[DOI] -
60. Sun W, Gregory DA, Tomeh MA, Zhao X. Silk fibroin as a functional biomaterial for tissue engineering. Int J Mol Sci. 2021;22(3):1499.
[DOI] -
61. Qi Y, Wang H, Wei K, Yang Y, Zheng RY, Kim IS, et al. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int J Mol Sci. 2017;18(3):237.
[DOI] -
62. Liu J, Yan L, Yang W, Lan Y, Zhu Q, Xu H, et al. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact Mater. 2019;4:151-159.
[DOI] -
63. Zhang F, Yin C, Qi X, Guo C, Wu X. Silk fibroin crosslinked glycyrrhizic acid and silver hydrogels for accelerated bacteria-infected wound healing. Macromol Biosci. 2022;22(4):2100407.
[DOI] -
64. Karahaliloglu Z, Ercan B, Denkbas EB, Webster TJ. Nanofeatured silk fibroin membranes for dermal wound healing applications. J Biomed Mater Res A. 2015;103(1):135-144.
[DOI] -
65. Kamalathevan P, Ooi PS, Loo YL. Silk-based biomaterials in cutaneous wound healing: a systematic review. Adv Skin Wound Care. 2018;31(12):565-573.
[DOI] -
66. Shen X, Zhang M, Bhandari B, Gao Z. Novel technologies in utilization of byproducts of animal food processing: A review. Crit Rev Food Sci Nutr. 2019;59(21):34203430.
[DOI] -
67. Al-Tayyar NA, Youssef AM, Al-Hindi R. Antimicrobial food packaging based on sustainable bio-based materials for reducing foodborne pathogens: A review. Food Chem. 2020;310:125915.
[DOI] -
68. Naomi R, Bahari H, Ridzuan PM, Othman F. Natural-based biomaterial for skin wound healing (Gelatin vs. collagen): Expert review. Polymers. 2021;13(14):2319.
[DOI] -
69. Cao H, Wang J, Hao Z, Zhao D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front Pharmacol. 2024;15:1398939.
[DOI] -
70. Ahmad MI, Li Y, Pan J, Liu F, Dai H, Fu Y, et al. Collagen and gelatin: Structure, properties, and applications in food industry. Int J Biol Macromol. 2024;254(3):128037.
[DOI] -
71. Colak B, Yormaz S, Ece I, Calisir A, Korez K, Cinar M, et al. Comparison of collagen granule dressing versus conventional dressing in patients with diabetic foot ulcer. Int J Low Extrem Wounds. 2022;21(3):279-289.
[DOI] -
72. Djavid GE, Tabaie SM, Tajali SB, Totounchi M, Farhoud A, Fateh M, et al. Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: A randomized control trial. J Wound Care. 2020;29(Sup3):S13-S18.
[DOI] -
73. Pinto NM. Case study in treatment of diabetic foot ulcer with alimentary gelatin. Br J Nurs. 2011;20(Sup2):S4-S8.
[DOI] -
74. Xu R, Fang Y, Zhang Z, Cao Y, Yan Y, Gan L, et al. Recent advances in biodegradable and biocompatible synthetic polymers used in skin wound healing. Materials. 2023;16(15):5459.
[DOI] -
75. Güiza-Argüello VR, Solarte-David VA, Pinzón-Mora AV, Ávila-Quiroga JE, Becerra-Bayona SM. Current advances in the development of hydrogel-based wound dressings for diabetic foot ulcer treatment. Polymers. 2022;14(14):2764.
[DOI] -
76. Mota JAD. Development of hydrogel dressings as delivery platforms for sustained and controlled delivery of miRNA for the treatment of diabetic foot ulcers [dissertation]. Barcelona (Spain): Universitat Ramon Llull; 2022.
-
77. Razif R, Fadilah NIM, Ahmad H, Looi Qi, Maarof M, Fauzi MB. Asiaticoside-loaded multifunctional bioscaffolds for enhanced hyperglycemic wound healing. Biomedicines. 2025;13(2):277.
[DOI] -
78. Salimi F, Mohammadipanah F. Nanomaterials versus the microbial compounds with wound healing property. Front Nanotechnol. 2021;2:584489.
[DOI] -
79. Atwal A, Dale TP, Snow M, Forsyth NR, Davoodi P. Injectable hydrogels: An emerging therapeutic strategy for cartilage regeneration. Adv Colloid Interface Sci. 2023;321:103030.
[DOI] -
80. Tejada Jacob G, Castro GR, Alvarez VA. Nanotechnology applied to personalized 3D dressings for diabetic feet. In: Mallakpour S, Hussain CM, editors. Handbook of Consumer Nanoproducts. Singapore: Springer; 2022. p. 525-547.
[DOI] -
81. Mehta S, Wadhwa S, Nayak SR, Kumar R. Hydrogel-based treatment strategies to accelerate diabetic foot ulcer healing. Curr Diabetes Rev. 2023;19(8):70-83.
[DOI] -
82. Wang S, Zhang J, Zhou W, Liu W, Ou Y, Zheng X, et al. Injectable carrier hydrogel for diabetic foot ulcer wound repair. J Mater Sci. 2023;58(28):11441-11468.
[DOI] -
83. Fang Y, Han Y, Yang L, Kankala RK, Wang S, Chen A, et al. Conductive hydrogels: Intelligent dressings for monitoring and healing chronic wounds. Regen Biomater. 2024;12:rbae127.
[DOI] -
84. Zhang Y, Wu BM. Current advances in stimuli-responsive hydrogels as smart drug delivery carriers. Gels. 2023;9(10):838.
[DOI] -
85. Hussain Z, Thu HE, Shuid AN, Katas H, Hussain F. Recent advances in polymer-based wound dressings for the treatment of diabetic foot ulcer: An overview of state-of-the-art. Curr Drug Targets. 2018;19(5):527-550.
[DOI] -
86. Li Z, Zhao Y, Liu H, Ren M, Wang Z, Wang X, et al. pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing. Mater Des. 2021;210:110104.
[DOI] -
87. Naghib SM, Amiri S, Mozafari M. Stimuli-responsive chitosan-based nanocarriers for drug delivery in wound dressing applications: A review. Carbohydr Polym Technol Appl. 2024;7:100497.
[DOI] -
88. Das IJ, Bal T. pH factors in chronic wound and pH-responsive polysaccharide-based hydrogel dressings. Int J Biol Macromol. 2024;279(1):135118.
[DOI] -
89. Wahid F, Zhao XJ, Jia SR, Bai H, Zhong C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos B Eng. 2020;200:108208.
[DOI] -
90. Mirani B, Pagan E, Currie B, Siddiqui MA, Hosseinzadeh R, Mostafalu P, et al. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv Healthc Mater. 2017;6(19):1700718.
[DOI] -
91. Renuka RR, Julius A, Yoganandham ST, Umapathy D, Ramadoss R, Samrot AV, et al. Diverse nanocomposites as a potential dressing for diabetic wound healing. Front Endocrinol. 2023;13:1074568.
[DOI] -
92. Jin S, Newton MA, Cheng H, Zhang Q, Gao W, Zheng Y, et al. Progress of hydrogel dressings with wound monitoring and treatment functions. Gels. 2023;9(9):694.
[DOI] -
93. Li Y, Xu T, Tu Z, Dai W, Xue Y, Tang C, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929-4943.
[DOI] -
94. Xu Y, Hu Q, Wei Z, Ou Y, Cao Y, Zhou H, et al. Advanced polymer hydrogels that promote diabetic ulcer healing: Mechanisms, classifications, and medical applications. Biomater Res. 2023;27(1):36.
[DOI] -
95. Wang L, Yang Y, Han W, Ding H. Novel design and development of Centella Asiatica extract-loaded poloxamer/ZnO nanocomposite wound closure material to improve anti-bacterial action and enhanced wound healing efficacy in diabetic foot ulcer. Regen Ther. 2024;27:92-103.
[DOI] -
96. Ranjit E, Hamlet S, George R, Sharma A, Love RM. Biofunctional approaches of wool-based keratin for tissue engineering. J Sci Adv Mater Devices. 2022;7(1):100398.
[DOI] -
97. Islam MR, Oyen ML. Mechanical characterization of hydrogels. In: Li H, Silberschmidt V, editors. The Mechanics of Hydrogels. Cambridge: Woodhead; 2022. p. 1-24.
[DOI] -
98. Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96-110.
[DOI] -
99. Li Q, Wang D, Jiang Z, Li R, Xue T, Lin C, et al. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front Chem. 2022;10:1038839.
[DOI] -
100. Yu Q, Qiao GH, Wang M, Yu L, Sun Y, Shi H, et al. Stem cell-based therapy for diabetic foot ulcers. Front Cell Dev Biol. 2022;10:812262.
[DOI] -
101. Cui J, Zhang S, Cheng S, Shen H. Current and future outlook of loaded components in hydrogel composites for the treatment of chronic diabetic ulcers. Front Bioeng Biotechnol. 2023;11:1077490.
[DOI] -
102. Loretelli C, Ben Nasr M, Giatsidis G, Bassi R, Lancerotto L, D’Addio F, et al. Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020;57:883-890.
[DOI] -
103. De Gregorio C, Contador D, Díaz D, Cárcamo C, Santapau D, Lobos-Gonzalez L, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11:168.
[DOI] -
104. Guo HS, Bai M, Zhu YN, Liu X, Tian S, Long Y, et al. Pro-healing zwitterionic skin sensor enables multi-indicator distinction and continuous real-time monitoring. Adv Funct Mater. 2021;31(50):2106406.
[DOI] -
105. Cho H, Blatchley MR, Duh EJ, Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267-288.
[DOI] -
106. Zhao L, Niu L, Liang H, Tan H, Liu C, Zhu F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl Mater Interfaces. 2017;9(44):37563-37574.
[DOI] -
107. Zhu S, Zhao B, Li M, Wang H, Zhu J, Li Q, et al. Microenvironment responsive nanocomposite hydrogel with NIR photothermal therapy, vascularization and anti-inflammation for diabetic infected wound healing. Bioact Mater. 2023;26:306-320.
[DOI] -
108. Zhu Y, Zhang J, Song J, Yang J, Du Z, Zhao W, et al. A multifunctional pro-healing zwitterionic hydrogel for simultaneous optical monitoring of pH and glucose in diabetic wound treatment. Adv Funct Mater. 2019;30(6):1905493.
[DOI] -
109. Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6):296-302.
[DOI] -
110. Jaehoon J, Lee S, Chang H, Minn KW. The pH value changes during wound healing process. J Korean Soc Plast Reconstr Surg. 2008;35(3):243-247. Available from:https://www.koreamed.org/SearchBasic.php?RID=2144714
-
111. Li Y, Qu X, Wang Q, Li S, Zhang Q, Zhang X. Tannic acid and carboxymethyl chitosan-based multi-functional double-layered hydrogel with pH-stimulated response behavior for smart real-time infection monitoring and wound treatment. Int J Biol Macromol. 2024;261:129042.
[DOI] -
112. Fan Y, Wang H, Wang C, Xing Y, Liu S, Feng L, et al. Advances in smart-response hydrogels for skin wound repair. Polymers. 2024;16(19):2818.
[DOI] -
113. Barnea Y, Weiss J, Gur E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag. 2010;6:21-27.
[DOI] -
114. Hu C, Long L, Cao J, Zhang S, Wang Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem Eng J. 2021;411:128564.
[DOI] -
115. Li Z, Chen Y, Wang X, Zhao Y, Liu Q, Zhang M, et al. Glucose and pH dual-responsive hydrogels with antibacterial, reactive oxygen species scavenging, and angiogenesis properties for promoting the healing of infected diabetic foot ulcers. Acta Biomater. 2024;190:205-218.
[DOI] -
116. Jaiswal R, Sherje AP. Recent advances in biopolymer-based smart hydrogel for wound healing. J Drug Deliv Sci Technol. 2024;99:105990.
[DOI] -
117. Zhang J, Hurren C, Lu ZT, Wang D. pH-sensitive alginate hydrogel for synergistic anti-infection. Int J Biol Macromol. 2022;222:1723-1733.
[DOI] -
118. Tang Y, Xu H, Wang X, Dong S, Guo L, Zhang S, et al. Advances in preparation and application of antibacterial hydrogels. J Nanobiotechnol. 2023;21(1):300.
[DOI] -
119. Hong Y, Xi Y, Zhang J, Wang D, Zhang H, Yan N, et al. Polymersome-hydrogel composites with combined quick and long-term antibacterial activities. J Mater Chem B. 2018;6:6311-6321.
[DOI] -
120. Yang Z, Huang R, Zheng B, Guo W, Li C, He W, et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv Sci. 2021;8(8):2003627.
[DOI]
Copyright
© The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite



