Yingnan Duan, School of Materials Science and Engineering, National Institute for Advanced Materials, Nankai University, Tianjin 300350, China. E-mail: ynduan@nankai.edu.cn
Ammonia (NH3), a critical chemical feedstock with a hydrogen content as high as 17.6 wt%, plays a vital role in both agricultural and industrial sectors while also being recognized as a promising carbon-free transportation fuel with significant potential[1-3]. Currently, industrial ammonia synthesis primarily relies on the Haber–Bosch process, which is energy-intensive and associated with substantial carbon dioxide emissions[4,5]. Thus, there is an urgent need to develop a low-carbon, mild ammonia synthesis pathway. Figure 1 shows the outlook of stages along a transition from the present-day Steam Methane Reforming-Haber Bosch (SMR-HB) process towards sustainable NH3, as well as NH3’s possible role in current and future markets. In recent years, the electrocatalytic nitrogen reduction reaction has emerged as a potential alternative strategy for ammonia synthesis. For example, Kartick et al. recently reported a Cu/Cu2O heterojunction catalyst that achieved efficient nitrate reduction under alkaline conditions, revealing the synergistic mechanism of Cu2O promoting nitrate deoxygenation, Cu promoting hydrogen adsorption and overflow, and achieving an ammonia Faraday efficiency of 85.62%.
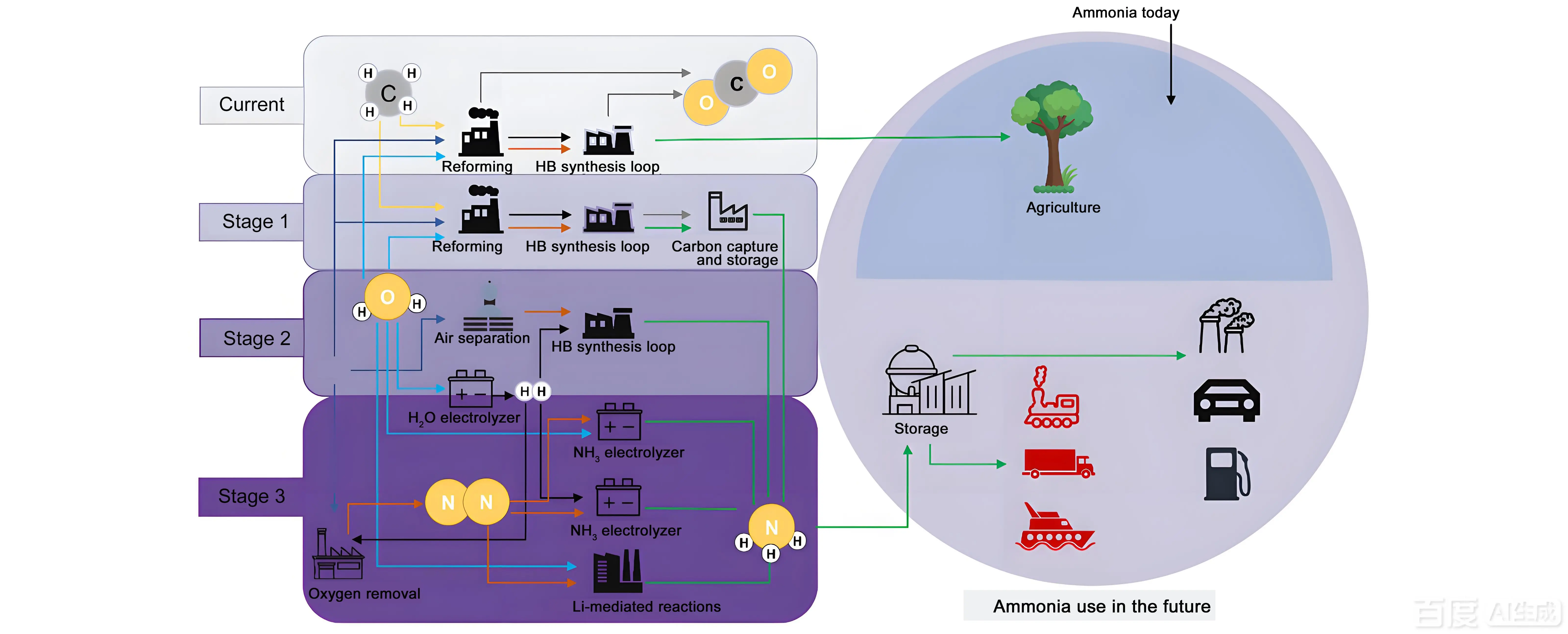
Figure 1. Outlook of stages along a transition from the present-day SMR-HB process towards sustainable NH3, as well as NH3’s possible role in current and future markets (each component of the schematic diagram was drawn by the author themselves). SMR-HB: Steam Methane Reforming-Haber Bosch.
However, its ammonia yield and Faraday efficiency remain generally low due to the extremely low solubility of nitrogen gas in water and the exceptionally high N≡N bond energy (941 kJ·mol-1)[6-9]. In contrast, nitrate ions (NO3-) exhibit higher water solubility and a lower N=O bond energy (only 236 kJ·mol-1), making them a more suitable raw material for electrolytic ammonia synthesis[10-14]. Furthermore, nitrate ions are widely present in domestic sewage and industrial wastewater, posing threats to aquatic ecosystems and human health. Therefore, the electrocatalytic reduction of nitrate ions to ammonia (NO3RR) can both alleviate environmental pollution and provide a sustainable ammonia synthesis pathway, offering dual advantages of environmental protection and energy conservation. However, most current research focuses on NO3RR processes involving low-concentration nitrates in alkaline or neutral media. Industrial nitrate-containing wastewater typically presents a strongly acidic, high-concentration environment. Under acidic conditions, high corrosion and competition from the hydrogen evolution reaction (HER) have led to limited catalyst research[15]. This implies that in practical applications, using catalysts designed for alkaline or neutral environments necessitate pre-neutralization of wastewater, leading to additional costs and energy consumption. This approach not only contradicts the objective reality of highly acidic, high-concentration industrial wastewater but also fails to align with the practical process requirements for future large-scale ammonia synthesis. Furthermore, industrial-grade acidic conditions facilitate product separation (such as the direct yield of ammonium salt fertilizers). However, due to the lack of highly active and stable catalysts, the utilization rate of hydrogen cannot be balanced with the adsorption kinetics of nitrate ions. Consequently, the issue of intense competition for the hydrogen evolution reaction under acidic, high-concentration conditions remains fundamentally unresolved.
Against this research backdrop, Yuan’s team published a high-impact report in Advanced Energy Materials. Using TiO2 as a substrate, the team designed a ruthenium-doped titanium dioxide (Ru-TiO2) nanosphere arrays as an ultra-stable, highly active acidic NO3RR electrocatalyst. This achievement provides highly valuable insights for addressing the research-industrialization gap in NO3RR applications[15]. As shown in Figure 2, the research team employed a one-step hydrothermal method. Pre-treated titanium foam was immersed in a mixed solution containing 9.8 mmol·L-1 RuCl3·3H2O and 0.05 mol·L-1 HCl, reacted at 200 °C for 20 hours, and then washed and dried to obtain the target product. Scanning electron microscopy (SEM) characterization revealed that this synthesis yields a well-defined array of uniform Ru-TiO2 nanospheres directly grown on the titanium foam substrate. This unique three-dimensional array architecture provides a high specific surface area, abundant active sites, and facilitates efficient mass transport, key factors contributing to its exceptional catalytic performance. Structural characterization revealed successful Ru atom doping into the TiO2 lattice, substituting tetracoordinate Ti sites and inducing approximately 2.85% lattice tensile strain. For comparative analysis, the team prepared Ru/TiO2 without lattice strain via impregnation, achieving similar Ru content but without TiO2 lattice expansion. Comparative analysis demonstrated that lattice strain effectively suppresses the dimerization of hydrogen species into H₂, thereby significantly inhibiting the competing HER and redirecting more active hydrogen toward the hydrogenation reduction pathway of nitrates. At the atomic scale, this suppression originates from the synergistically regulated H adsorption behavior by Ru doping: electronic structure optimization reduces the adsorption energy of H, preventing its excessive binding, while lattice strain further impedes H₂ formation by increasing the energy barrier for surface H migration and dimerization. This dual-mechanism synergy enables H to more readily participate in the stepwise hydrogenation process of NO3-, thereby achieving highly selective ammonia synthesis under strongly acidic conditions. The team further confirmed via X-ray photoelectron spectroscopy and electron density distribution calculations that Ru introduction induces significant electron transfer from Ti to Ru, optimizing the catalyst’s d-band center. This enhances adsorption of nitrogen-containing intermediates and lowers the reaction energy barrier. Yuan’s team achieved synergistic effects of electronic structure regulation and lattice strain engineering through a single-step doping process.
Under near-extreme acidic and high-salinity conditions (pH = 1, 6M NO3-), Yuan’s team comprehensively validated the integrated performance of Ru-TiO2 nanosphere arrays through rigorous testing[15]. Figure 3 shows the comparative performance of the Ru-TiO2 catalyst with other reported catalysts. Linear voltammetry and Tafel slope measurements revealed significantly enhanced reaction current and accelerated kinetics, demonstrating the Ru-TiO2 nanosphere arrays outstanding intrinsic activity. Chronoamperometry demonstrated that Ru-TiO2 nanosphere arrays achieved 98.8% ammonia Faradaic efficiency and an ammonia production rate of 69.6 mg·h-1·cm-2 at -0.9 V vs. RHE, proving their high efficiency and productivity under extreme conditions. Isotope labeling experiments combined with ¹H NMR confirmed that the produced ammonia originated entirely from nitrate reduction. Simultaneously, byproduct detection indicated negligible N2H4 formation, verifying the Ru-TiO2 nanosphere arrays catalyst’s exceptionally high ammonia selectivity; Long-term stability testing demonstrated that Ru-TiO2 nanosphere arrays maintained a Faraday efficiency of approximately 94.5% after 300 hours of continuous operation at industrial-scale current density (approximately -860 mA·cm-2), with no significant structural changes, confirming their outstanding long-term stability. ICP-OES analysis of the post-reaction electrolyte revealed extremely low leaching of Ru and Ti elements, verifying their structural robustness. The outstanding activity, selectivity, long-term stability, and structural robustness exhibited by Ru-TiO2 nanosphere arrays under extreme testing conditions prompted us to conduct industrialization-oriented research on Ru-TiO2 nanosphere arrays.
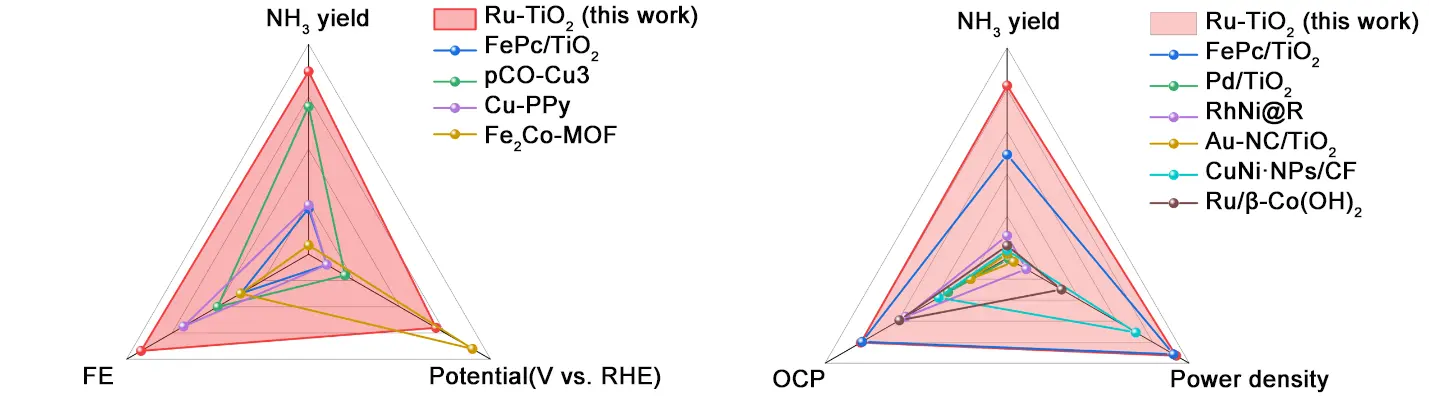
Figure 3. Comparisons of NO3RR performances between Ru-TiO2 and recently literature-reported catalysts under acidic conditions, and Comparison of the Ru-TiO2-based AAZNB with the reported Zn-NO3- batteries. AAZNB: alkaline-acid hybrid zinc-nitrate battery; FE: Faraday efficiency; NO3RR: nitrate reduction reaction; OCP: open-circuit potential; RHE: reversible hydrogen electrode.
Yuan’s team further utilized Ru-TiO2 nanosphere arrays to assemble a novel alkaline-acid hybrid zinc-nitrate battery (AAHZNB), achieving an integrated ‘wastewater treatment-ammonia synthesis-power generation’ system[15]. The design principle of this battery is based on the ingenious conception of an acid-base dual electrolyte architecture: the Ru-TiO2 nanosphere array serves as the cathode in an acidic electrolyte to optimize NO3RR and directly yield ammonium salts, while a zinc plate acts as the anode in an alkaline electrolyte to promote zinc oxidation and suppress hydrogen evolution. The two compartments are separated by an ion-exchange membrane, which prevents crossover of reactants while allowing selective migration of essential ions such as H+ to maintain charge balance. During operation, the cathode undergoes NO3- reduction to NH3, while the anode undergoes zinc oxidation. These reactions synergistically drive battery discharge. The cell exhibits an open-circuit voltage as high as 2.01 V, with a peak power density reaching 92.8 mW·cm-2, surpassing all previously reported zinc-nitrate batteries. Regarding long-term cycling stability, during continuous 10-hour discharge testing over 10 cycles, the battery maintained over 93% ammonia Faraday efficiency at a current density of -97.8 mA·cm-2 while stably producing 20.5 mg·h-1·cm-2 ammonia, and the discharge curves retained a stable ladder like shape without significant performance decay. Figure 3 also shows the comparison between Ru-TiO2-based AAZNB and reported Zn-NO3- batteries. It is not difficult to see that Ru-TiO2-based AAZNB exhibits excellent Faraday efficiency, power density, NH3 yield, and open circuit voltage. This outstanding performance further demonstrates the practical value of Ru-TiO2 nanosphere arrays in electrochemical ammonia production and energy conversion technologies.
Overall, Yuan’s team successfully developed Ru-TiO2 nanosphere arrays with lattice strain for NO3RR under industrial acidic conditions, demonstrating outstanding NH3 production performance and exceptional stability at industrial current densities. This research aligns with the objective environment of high-concentration acidic industrial wastewater while meeting future process demands for large-scale ammonia synthesis. Furthermore, the team innovatively assembled a novel AAHZNB using Ru-TiO2 nanosphere arrays, enabling simultaneous NH3 and electricity generation. This work provides valuable references for subsequent industrialization studies of NO3RR. Yuan’s team has successfully bridged fundamental research with practical applications. This synergistic design strategy, combined with novel research methods and paradigms such as machine learning and artificial intelligence[16,17], is poised to accelerate the discovery of next-generation electrocatalysts that perform optimally in complex real-world environments.
Author contribution
Li Y: Conceptualization, data curation, formal analysis, writing-original draft.
Lei Y: Data curation, formal analysis, writing-original draft.
Yang Y: Data curation, visualization, writing-original draft.
Duan Y, Shen Z: Conceptualization, supervision, writing-review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2023YFC3708005) and the National Natural Science Foundation of China (Grant Nos. 21872102 and 22172080).
Copyright
© The Author(s) 2025.
References
-
1. Chen GF, Yuan YF, Jiang HF, Ren SY, Ding LX, Ma L, et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst. Nat Energy. 2020;5(8):605-613.[DOI]
-
2. Sun ML, Wang HY, Feng Y, Ren JT, Wang L, Yuan ZY. Electrodegradation of nitrogenous pollutants in sewage: from reaction fundamentals to energy valorization applications. Chem Soc Rev. 2024;53(24):11908-11966.[DOI]
-
3. Xiong YC, Wang YH, Zhou JW, Liu F, Hao FK, Fan ZX. Electrochemical nitrate reduction: Ammonia synthesis and the beyond. Adv Mater. 2024;36(17):2304021.[DOI]
-
4. Zhang S, Wu J, Zheng M, Jin X, Shen Z, Li Z, et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat Commun. 2023;14(1):3634.[DOI]
-
5. Feng Y, Lv X, Wang H, Wang H, Yan F, Wang L, et al. *H species regulation of heterostructured Cu2O/NiO nanoflowers boosting tandem nitrite reduction for high-efficiency ammonia production. Adv Funct Mater. 2025;35(34):2425687.[DOI]
-
6. Chu K. Lewis Acid Fe-V pairs promote nitrate electroreduction to ammonia. Adv Funct Mater. 2024;34(14):2312801.[DOI]
-
7. Xiang J, Qiang C, Shang S, Chen K, Kang C, Chu K. Tandem electrocatalytic reduction of nitrite to ammonia on rhodium–copper single atom alloys. Adv Funct Mater. 2024;34(36):2401941.[DOI]
-
8. Zhu G, Bao W, Xie M, Qi C, Xu F, Jiang Y, et al. Accelerating tandem electroreduction of nitrate to ammonia via multi-site synergy in mesoporous carbon-supported high-entropy intermetallics. Adv Mater. 2025;37(5):2413560.[DOI]
-
9. Majhi KC, Le Q, Wu Z, Xu X, Jin Y, Shao S, et al. Unveiling the reaction mechanism of electrochemical nitrate reduction to ammonia on a Cu/Cu2O heterojunction catalyst. Energy Fuels. 2025;39(33):15903-15911.[DOI]
-
10. Wang HY, Ren JT, Sun ML, Tian WW, Feng Y, Yuan ZY. Value-added aqueous metal-redox bicatalyst batteries. Adv Energy Mater. 2024;14(2):2302515.[DOI]
-
11. Yan Q, Zhao R, Yu L, Zhao Z, Liu L, Xi J. Enhancing compatibility of two-step tandem catalytic nitrate reduction to ammonia over P-Cu/Co(OH)2. Adv Mater. 2024;36(45):2408680.[DOI]
-
12. Feng Y, Ren JT, Song YX, Tian WW, Wang HY, Wang L, et al. Super-hydrophilic/aerophobic Co(OH)2/NiFe layered double hydroxide heterostructures with moderate work function difference for large-current-density electrochemical ammonia synthesis. CCS Chem. 2025;7(5):1344-1358.[DOI]
-
13. Zhang Z, Li D, Tu Y, Deng J, Bi H, Yao Y, et al. Electrocatalytic synthesis of C–N coupling compounds from CO2 and nitrogenous species. SusMat. 2024;4(2):e193.[DOI]
-
14. Wang M, Khan MA, Mohsin I, Wicks J, Ip AH, Sumon KZ, et al. Can sustainable ammonia synthesis pathways compete with fossil-fuel based haber–bosch processes? Energy Environ Sci. 2021;14(5):2535-2548.[DOI]
-
15. Feng S, Lv X, Wang H, Wang L, Sun M, Ren J, et al. The combination of electronic structure and lattice strain engineering for ultra-stable acidic nitrate electroreduction at highly concentrated electrolyte. Adv Energy Mater. 2025;15(38):e03022.[DOI]
-
16. Zhu R, Wang H, Tang K, Yang X, Zhao X, Yu J, et al. Single-atom collaboration with cluster for accelerated nitrate electroreduction: Synergy revelation via machine learning and DFT calculations. J Energy Chem. 2026;112:852-860.[DOI]
-
17. Chang G, Chen X, Lv JJ, Kong Z, Wang ZJ. Cobalt-based electrocatalysts for sustainable nitrate conversion: Structural design and mechanistic advancements. Nano-Micro Lett. 2026;18(1):73.[DOI]
Copyright
© The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite