Eryang Mao, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China. E-mail: maoeryang@foxmail.com
Abstract
Electrocatalytic water splitting is a key technology for sustainable hydrogen production, but its efficiency is limited by the slow kinetics of the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). Spinel oxides (AB2O4) have drawn significant interest due to their electrochemical stability, tunable electronic properties, and low cost. Their catalytic performance is highly influenced by the crystalline phase, which governs charge transport, active site density, and reaction energetics. However, the effects of phase transitions and structural variations on electrocatalysis remain insufficiently explored. This review systematically evaluates the performance of spinel oxides across six primary crystal phases: cubic, hexagonal, tetragonal, orthorhombic, rhombohedral, and monoclinic, discussing how these structural features affect charge transport, intermediate adsorption, and reaction energetics. The review emphasizes the structure-electronic-catalytic performance relationships and covers phase variants, including coordination environment modulation, defect regulation, metastable phase transitions, and strain engineering. It further examines how phase symmetry, oxygen coordination, orbital rearrangement, and interfacial characteristics influence OER and HER kinetics. Challenges in phase engineering, such as controlling phase transitions and stabilizing metastable phases, are also highlighted. This review provides a framework for understanding the electrocatalytic behavior of spinel oxides and offers guidance for designing high-performance catalysts for water splitting.
Keywords
1. Introduction
Electrocatalytic water splitting, as a promising technology for hydrogen production, is widely regarded as a key strategy to address the global energy transition[1]. This process efficiently converts water into hydrogen and oxygen, providing a crucial solution for the advancement of clean energy. In the context of the ongoing transformation of global energy structures, the development of efficient, stable, and low-cost electrocatalysts, particularly for optimizing the catalytic performance in the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), has become a central challenge to enhance the efficiency of water electrolysis[2,3]. Spinel oxides (AB2O4), due to their excellent electrochemical stability, diverse composition, and relatively low cost, have emerged as promising alternatives to precious metal-based catalysts[4]. They exhibit superior durability in extreme electrochemical environments and can sustain efficient catalytic performance during large-scale hydrogen production processes. Particularly, transition metal ions at the B-site (e.g., Ni, Co, Fe, and Cu) have tunable oxidation-reduction properties, offering a broad potential for optimizing catalytic activity[5,6]. However, despite the advantages in stability, the electrocatalytic performance of spinel oxides is still limited by several inherent challenges. First, spinel oxides typically exhibit low surface area and limited active sites, which restrict their catalytic efficiency in OER and HER[7]. Specifically, in alkaline electrolytes, their charge transfer kinetics and conductivity often fail to meet the demands of high current density, posing a significant bottleneck in practical applications[8,9]. Second, the activation efficiency of reaction intermediates is relatively low, leading to suboptimal reaction pathways and further reducing overall water splitting performance. Although surface defects, such as oxygen vacancies, have shown potential in improving catalytic performance, their precise role, especially in accelerating reaction kinetics and facilitating intermediate adsorption, remains inadequately understood[10,11]. Therefore, addressing these structural and electronic bottlenecks through a deeper understanding of the spinel oxides’ catalytic behavior in various crystal phases and structural variants is essential for advancing their application in electrocatalytic water splitting[12,13].
Spinel oxides exhibit a range of crystal phases, each of which presents distinct structural and electronic properties that influence their catalytic performance in electrocatalytic water splitting[14,15]. The conventional cubic spinel structure, with its high symmetry and stable lattice configuration, provides ideal charge transfer channels and catalytic active sites, making it a commonly used base material for catalysis[16,17]. However, as research advances, increasing evidence suggests that non-cubic spinel phases, such as hexagonal, tetragonal, and metastable phases, can significantly enhance catalytic performance through various lattice distortions and defect modulations[18]. For instance, hexagonal and tetragonal spinel oxides demonstrate higher activity in the OER due to their lower crystal symmetry and defect-rich structures, which facilitate efficient intermediate adsorption and optimization of reaction pathways[19,20]. Furthermore, metastable spinel phases, with their unique structural characteristics and dynamic electronic behaviors, offer more reaction sites and improve reaction kinetics, particularly in the HER, where they exhibit higher catalytic activity[21]. Despite these promising characteristics, several challenges remain in realizing the full potential of non-cubic spinel oxides[22,23]. First, precisely controlling the synthesis conditions of these non-cubic phases and ensuring their stability during electrocatalytic reactions remains a critical challenge. Metastable phases often exist at higher energy states, making them prone to phase transitions under practical reaction conditions, resulting in fluctuating and unstable catalytic performance[24,25]. Additionally, the phase transformation dynamics are complex, and the heterogeneity between different phases may lead to interface mismatches, reducing catalytic efficiency[26,27]. Another challenge lies in the fact that while surface defects have been shown to enhance catalytic performance, the systematic understanding of how these defects optimize catalytic activity is still lacking[28,29]. Most current research is focused on single-phase systems or specific material types, without a comprehensive structural perspective on how different spinel phases and their variants influence catalytic mechanisms[30,31]. Therefore, further exploration of precise structural modulation and phase-engineering strategies, together with a deeper investigation of how structural features govern electronic states and ultimately catalytic performance, is essential for overcoming the inherent performance limitations of spinel oxides[32,33].
This review aims to comprehensively assess the potential of spinel oxides in electrocatalytic water splitting from a crystallographic perspective, shedding light on the deep relationships between different crystal phases and their catalytic performance. Unlike traditional studies that focus on single-phase or specific material systems, this review systematically discusses the six main crystal phases of spinel oxides (cubic, hexagonal, tetragonal, orthorhombic, rhombohedral, and monoclinic) and their variants, highlighting their distinct impacts on electrocatalytic water splitting performance (Figure 1). It provides an at-a-glance roadmap of this review, linking the major spinel crystal phases and phase-variant regulation strategies to their roles in lattice-coupled OER/HER catalysis and broader energy-conversion applications. By analyzing the lattice symmetry, oxygen coordination environment, orbital rearrangement, and interface properties of these phases, this work provides a comprehensive framework to help readers understand how different crystal structures govern the electrocatalytic behavior of spinel oxides, particularly in OER and HER catalytic dynamics. The novelty of this review lies in its integration of how distinct crystal phases govern electrocatalytic water splitting through a phase-specific classification framework, thereby elucidating the intrinsic coupling among structural features, electronic configurations, and catalytic performance. Through a systematic analysis of how different phases influence charge transfer efficiency, intermediate adsorption, and reaction energy barrier optimization, this review offers new insights for the design of highly efficient electrocatalysts for water splitting. Furthermore, this review addresses the precise control of phase transitions, the stability of metastable phases, and the impact of phase heterogeneity on catalytic performance, proposing key directions for future research. Overall, this work aims to establish a systematic framework for advancing spinel oxide catalysts and guide the future development of high-performance materials for sustainable water electrolysis.
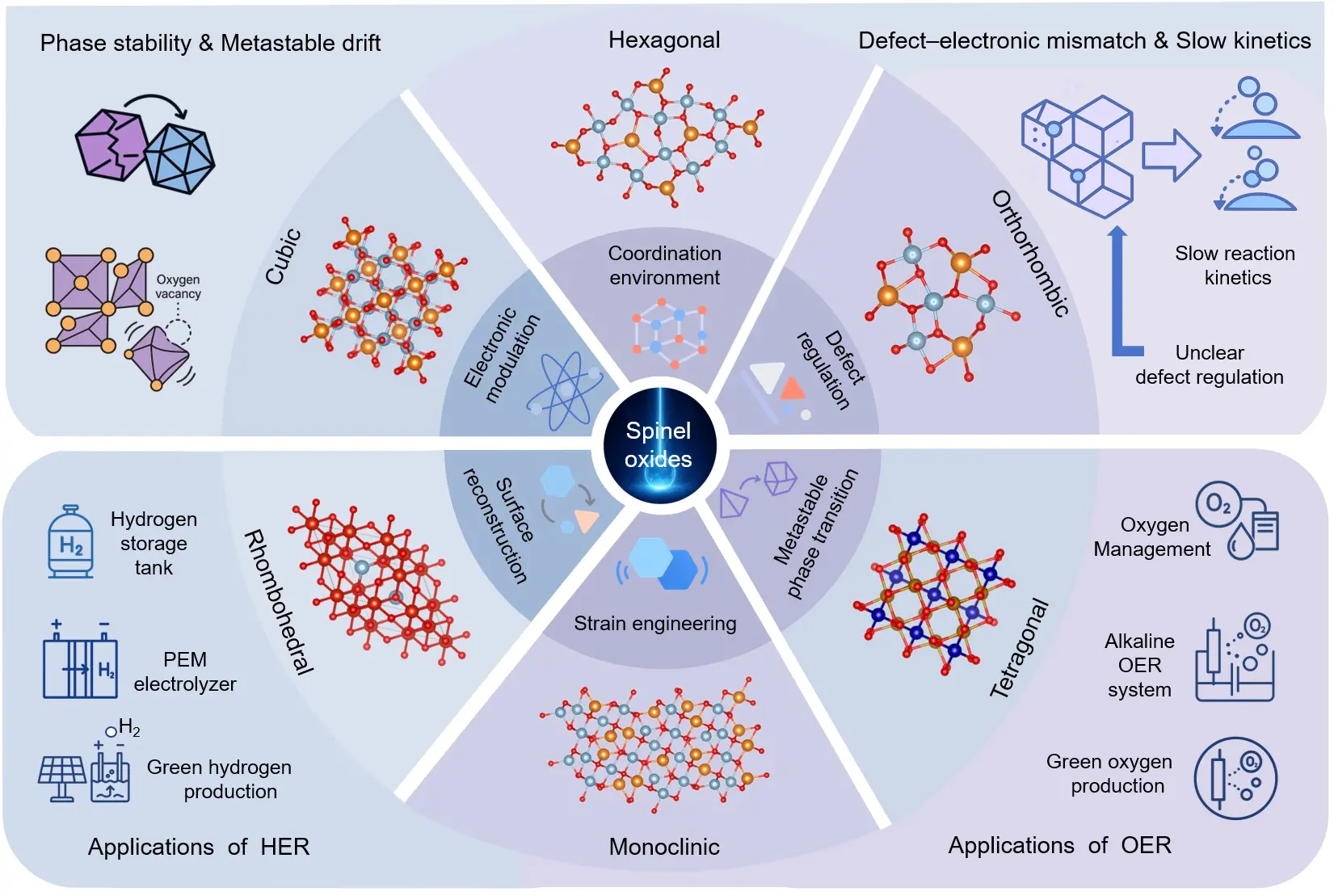
Figure 1. Structural phases, regulation strategies, and energy-conversion applications of spinel oxides.
2. Fundamental Principles and Mechanisms Governing Spinel Oxides for Electrocatalytic Water Splitting
Spinel oxides are widely used in electrocatalytic water splitting due to their excellent electrochemical stability, tunable electronic structures, and abundant active sites. The unique characteristics of the spinel structure contribute to superior performance in charge transport, oxygen vacancy formation, and reaction kinetics, while also ensuring long-term catalytic stability[34,35]. To further enhance their catalytic performance, a comprehensive understanding of the fundamental catalytic mechanisms and structural features of spinel oxides is crucial. In this review, “lattice-coupled water-redox electrocatalysis” denotes a bidirectional coupling in which the catalyst lattice (symmetry/coordination, defects/strain, cation distribution) sets the electronic structure and adsorption energetics for OER/HER, while operating potentials/intermediates can in turn trigger lattice responses (vacancies, cation redistribution, surface reconstruction/phase evolution). This section first explores the underlying principles and advantages of spinel oxides in water splitting, followed by an in-depth analysis of the six major crystal phases of spinel oxides, including cubic, hexagonal, orthorhombic, tetragonal, rhombohedral, and monoclinic, with a focus on how their structural characteristics influence catalytic performance[36,37]. It then discusses the impacts of structural variants, such as coordination environment modulation, defect regulation, metastable phase transitions, and strain engineering. Finally, a summary and analysis of existing research studies are provided, revealing the structure–performance relationships of spinel oxides and offering theoretical guidance for the rational design of high-performance spinel oxide electrocatalysts[38,39].
2.1 Advantages and fundamental mechanisms of spinel oxides for electrocatalytic water splitting
Spinel oxides have attracted significant attention in electrocatalytic water splitting due to their exceptional electrochemical stability, tunable electronic structure, and abundant active sites. These properties enable spinel oxides to exhibit superior catalytic performance in water splitting reactions[40,41]. The AB2O4 consists of metal ions at A and B sites, along with oxygen ions. The A-site typically contains metals with high oxidation states, such as aluminum, magnesium, and zinc, while the B-site hosts transition metals like cobalt, nickel, iron, and copper[42]. Variations in the coordination environment, electronic properties, and ionic radii of these metal ions influence not only catalytic performance but also charge transfer, oxygen vacancy formation, and the adsorption and conversion of reaction intermediates[43,44]. These distinctive structural features make spinel oxides highly promising catalysts for electrocatalytic water splitting.
From a fundamental mechanism perspective, the electrocatalytic performance of spinel oxides is closely linked to their crystalline structure. The high symmetry and stable lattice configuration of spinel oxides facilitate the formation of a high density of active sites, promoting efficient charge transfer[45]. In the OER, the introduction of oxygen vacancies enhances reaction rates by modifying the electronic structure and strengthening the adsorption of intermediates[46,47]. The presence of oxygen vacancies facilitates electron migration and provides additional adsorption sites for oxygen molecules, thus accelerating oxygen generation. In the HER, structural defects and the reduction states of transition metal ions at the B-site play critical roles. The electronic density and redox properties of these metal ions directly impact the rate of hydrogen generation[48]. By adjusting the types of B-site metal ions, the density of oxygen vacancies, and the state of surface defects, the catalytic activity can be significantly improved, optimizing the energy pathway for hydrogen generation during water splitting[49].
Furthermore, spinel oxides offer distinct advantages in terms of their superior stability and low cost compared to precious metal catalysts. Spinel oxides uniquely combine a robust 3D close-packed lattice with dual-site (A/B) cation tunability, enabling phase/defect engineering while maintaining high durability at practically relevant current densities. Unlike noble metal catalysts, spinel oxides maintain long-term stable performance even at high current densities, making them ideal for large-scale applications[50]. Additionally, their relatively low synthesis costs make them more feasible for widespread use in industrial applications. The abundant availability and low cost of transition metal oxides further enhance the sustainability and economic viability of spinel oxides as electrocatalysts. These advantages provide a solid foundation for their application in large-scale water electrolysis systems[51,52].
2.2 Crystal structure of spinel oxides for classification and structural insights
The six main crystal phases of spinel oxides, including cubic, hexagonal, tetragonal, orthorhombic, rhombohedral, and monoclinic, each exhibit unique structural features that, to a large extent, govern their performance in electrocatalytic water splitting (Figure 2). Here, the classification adopted in this section follows a symmetry-lowering progression, where changes in coordination geometry, lattice distortion, and vacancy ordering collectively reshape the electronic structure and thus electrocatalytic activity[53]. Accordingly, phase symmetry, the coordination environment of metal ions, the distribution of oxygen vacancies, and lattice distortions regulate the electronic structure of spinel oxides, subsequently affecting catalytic reaction efficiency and selectivity[54,55]. By analyzing the structural characteristics of these phases, a better understanding can be gained of how spinel oxides perform in various electrocatalytic reactions, providing a theoretical basis for their optimization. Therefore, a detailed exploration of the classification of different crystal phases and their structural features will offer a more systematic theoretical framework for subsequent catalytic performance studies and material design. Unlike prior reviews organized by composition, dimensionality/morphology, or specific applications (OER/HER), we treat the crystal phase (symmetry) as the first-order design variable and unify doping, defects, and morphology as within-phase variants. The lattice-coupled view further emphasizes operando phase/valence evolution as a mechanistic determinant of activity-stability trade-offs.
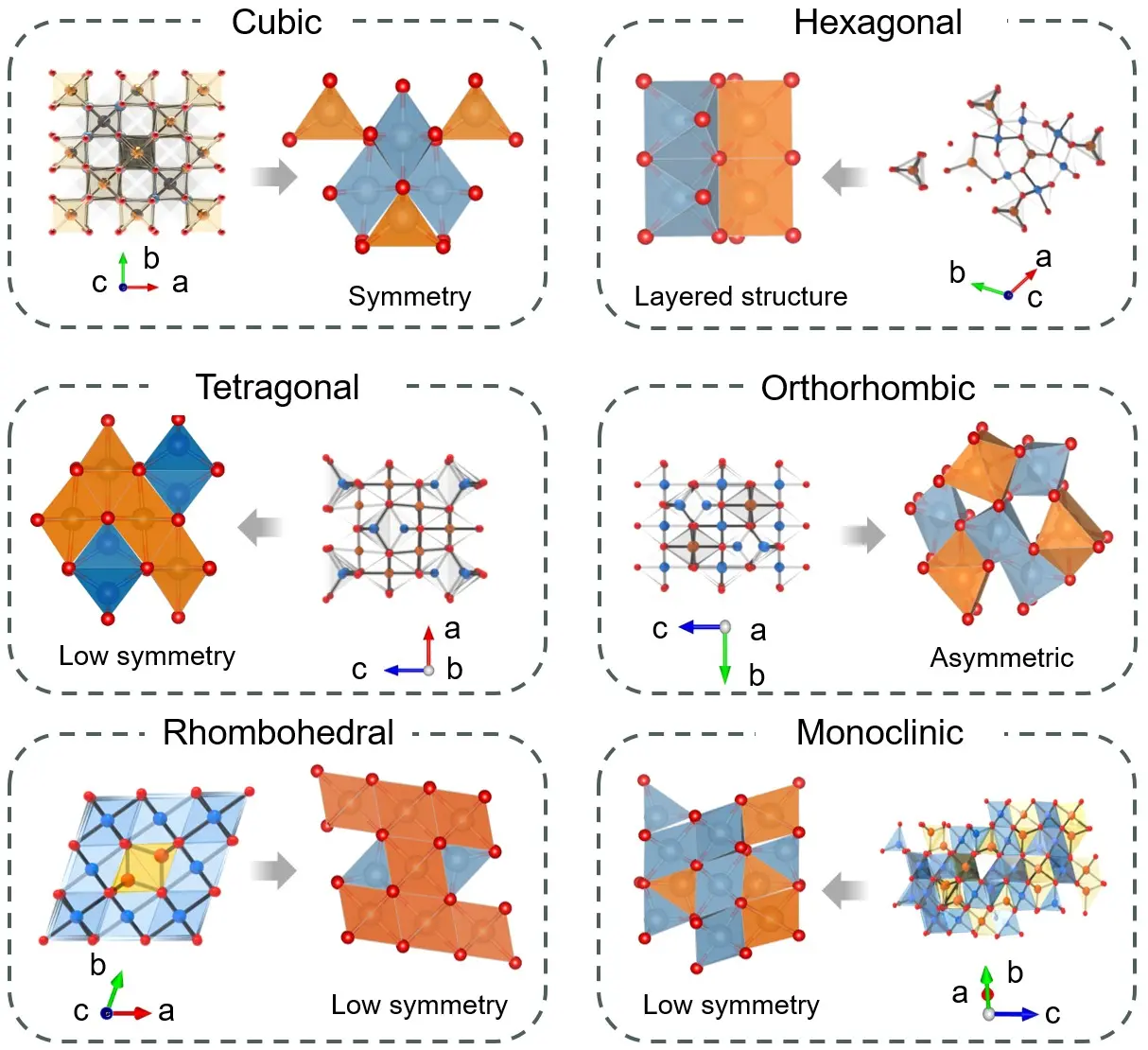
Figure 2. Representative cubic-to-triclinic phases illustrate symmetry evolution, coordination geometry, lattice distortion, and vacancy ordering, and their roles in determining the electronic structure and electrocatalytic activity.
2.2.1 Cubic phase
The cubic spinel oxide structure exhibits a face-centered cubic (FCC) symmetry, with two types of metal ions, A and B, occupying tetrahedral and octahedral positions, respectively, within the unit cell. Specifically, the larger metal ions, often Ni2+ or Co2+, occupy the tetrahedral positions, while the smaller metal ions, such as Fe3+ or Cr3+, are located in the octahedral sites[56,57]. Oxygen ions are positioned at the face centers and corners of the unit cell, forming a dense three-dimensional network of oxygen ions. This arrangement results in high symmetry within the cubic spinel structure, facilitating efficient charge transfer and contributing to enhanced conductivity and catalytic activity due to its simplified charge transport pathways[58,59]. In this structure, strong charge interactions between the A and B site metal ions are mediated by the oxygen ions, which play a crucial role in both lattice stability and charge conduction. The coordination environment around the B-site metal ions, which form octahedral coordination with oxygen ions, is critical in determining the oxidation-reduction behavior of the metal ions[60]. The typical bond distances between the oxygen ions and metal ions in the octahedral coordination are generally in the range of 1.9-2.2 Å[61]. This specific coordination environment not only influences the metal ions' redox behavior but also governs charge migration and intermediate adsorption during catalysis. The high symmetry and uniform distribution of oxygen ions in the cubic spinel structure also contribute to the material's thermodynamic and kinetic stability[62,63]. Furthermore, the cubic spinel oxide structure typically exhibits low internal stress and high structural stability, which can be attributed to the high symmetry and the well-ordered metal-oxygen coordination. The formation of oxygen vacancies in this structure is relatively flexible, with vacancies primarily occurring near the octahedral B-sites[64,65]. The introduction of oxygen vacancies improves the uniform distribution of charge and enhances both conductivity and catalytic activity. The stability of the cubic spinel structure is further reinforced by the formation of a stable three-dimensional oxygen ion network, which helps maintain the integrity of the structure across varying temperatures and in different acidic or alkaline environments[66]. In summary, the unique crystal structure of cubic spinel oxides imparts exceptional performance in electrocatalytic applications. Its face-centered cubic symmetry, precise metal ion coordination, and ordered oxygen ion arrangement provide high stability and excellent performance in charge transfer and catalytic reactions[67,68].
2.2.2 Hexagonal phase
Hexagonal spinel oxides belong to the hexagonal crystal system, with their unit cell composed of A- and B-site metal cations and oxygen anions, exhibiting a layered structure[69,70].In contrast to the cubic phase, hexagonal spinels exhibit lower symmetry, where A-site metal cations predominantly occupy octahedral sites, while B-site metal cations are primarily distributed in tetrahedral sites[71]. The oxygen anions are arranged in a bilayer stacking pattern within the unit cell, with one layer surrounding the octahedral A-site metal cations, and the other positioned near the tetrahedral B-site cations. In hexagonal spinels, the A-site metal cations have a stronger octahedral coordination, while the B-site cations exhibit looser coordination[72,73]. The metal-oxygen bond lengths typically range between 2.0 and 2.4 Å. This elongated coordination bond enhances electron transfer during redox reactions. Due to the lower symmetry, the electronic structure of the B-site metal cations is more complex, which can further influence the adsorption of reaction intermediates and optimize reaction pathways[74,75]. The lattice distortion in hexagonal spinels creates the potential for oxygen vacancy formation, which typically occurs around the octahedral B-site metal cations. Oxygen vacancies not only facilitate electronic conductivity but also improve the material's stability under extreme conditions[76]. The bilayer oxygen anion stacking also provides more space for oxygen ion migration and metal cation exchange, enhancing performance under high-temperature or high-current-density conditions. The stability of hexagonal spinels is highly dependent on the regulation of lattice distortions[77]. The lower symmetry makes these materials more susceptible to local structural changes or phase transitions under high-temperature or high-current-density conditions. However, by optimizing synthesis conditions, the stability of the hexagonal phase can be improved, preventing unnecessary phase transformations[78]. In conclusion, the unique crystalline architecture of hexagonal spinel oxides, defined by their low structural symmetry, layered stacking motif, intricate metal-oxygen coordination environments, and intrinsic oxygen vacancy formation, provides substantial opportunities for rationally tuning their catalytic performance[79,80].
2.2.3 Tetragonal phase
The tetragonal spinel oxide belongs to the tetragonal crystal system, with structural characteristics that differ notably from the cubic and hexagonal phases, exhibiting relatively lower symmetry. In this structure, A-site metal ions are typically positioned in octahedral coordination, while B-site metal ions are in tetrahedral coordination[81,82]. Compared to the cubic phase, the oxygen ion arrangement in the tetragonal phase is distorted, with oxygen-oxygen distances generally ranging from 2.2 to 2.4 Å, deviating significantly from the regular arrangement seen in the cubic phase[83,84]. This alteration in coordination leads to changes in the electron density around the metal ions, which subsequently influences charge transport and the interactions with reactants. The lattice structure of tetragonal spinel oxides exhibits slight axial or lateral elongation, causing variations in the distance between the A-site and B-site metal ions[85]. Due to the lower symmetry of the tetragonal crystal structure, the position of metal ions in the coordination environment is more susceptible to distortion, particularly under reaction conditions, which may result in local lattice strain. The oxygen-metal-oxygen bond length typically ranges from 2.1 to 2.3 Å, with a slight elongation compared to the cubic phase[86,87]. These changes not only affect the coordination environment of the metal ions but also contribute to the formation of oxygen vacancies and the introduction of lattice defects, which in turn modify the electronic structure and catalytic activity of the phase[88]. Moreover, due to the lower symmetry of the tetragonal phase, local structural distortions are more likely to occur during synthesis or operation, particularly under high current density or high-temperature conditions[89,90]. Although the overall lattice shows good stability, slight distortions in the structure may alter the coordination environment, leading to performance fluctuations under different conditions. Overall, while tetragonal spinel oxides exhibit relatively high stability, structural distortions pose a challenge. To ensure the material’s stability and reliability under various reaction conditions, it is necessary to optimize the synthesis process and conditions, minimizing local strain in the lattice[91].
2.2.4 Orthorhombic phase
Orthorhombic spinel oxides belong to the orthorhombic crystal system, exhibiting low crystal symmetry and a complex lattice configuration. In this structure, A-site metal ions typically occupy octahedral positions, while B-site metal ions are distributed between tetrahedral and octahedral sites, creating an asymmetric coordination environment[92,93]. This non-symmetric coordination results in significant lattice distortion and internal stress. Oxygen ions in the crystal do not exhibit symmetry, typically adopting two different coordination modes, which further complicates the structure[94]. The coordination distance between A-site metal ions and oxygen ions is typically in the range of 2.1 to 2.3 Å, while the distance between B-site metal ions and oxygen ions is slightly larger, usually between 2.3 and 2.5 Å[94]. Due to the differing coordination environments of the metal ions, the interactions between A-site and B-site metal ions are complex, leading to more varied electron density distributions and charge transfer pathways[95]. This lattice distortion influences the conductivity and catalytic behavior. The lattice constants a, b, and c in the orthorhombic phase exhibit anisotropy, with the c-axis typically exhibiting more significant elongation, indicating notable deformation along different lattice[96]. The arrangement of oxygen ions in orthorhombic spinel oxides forms a relatively loose metal-oxygen-metal network. The irregular coordination and variations in the positioning of oxygen ions introduce significant lattice strain, which may lead to the formation of local defects or further lattice distortion, particularly under high-temperature or high-current density conditions[97,98]. Overall, the structural characteristics of orthorhombic spinel oxides are defined by considerable lattice distortion and the asymmetric coordination of metal-oxygen sites, providing considerable flexibility for material performance tuning while also presenting stability challenges[99].
2.2.5 Rhombohedral phase
Rhombohedral spinel oxides belong to the rhombohedral crystal system, characterized by lower symmetry and a more complex coordination environment compared to other phases. In this structure, A-site metal ions typically occupy octahedral coordination, while B-site metal ions are primarily distributed between tetrahedral and octahedral sites, forming an asymmetric coordination[100]. This asymmetry leads to significant lattice strain, and the arrangement of oxygen ions within the unit cell is distorted, adding to the complexity of the structure[101]. The coordination distance between A-site metal ions and oxygen ions typically ranges from 2.3 to 2.5 Å, while the distance between B-site metal ions and oxygen ions is in the range of 2.1 to 2.3 Å[102]. Due to the different coordination environments of the A and B metal ions, the distribution of electron density and charge transport characteristics within the lattice exhibit variations. The complex interactions between A and B metal ions further complicate the electron transfer pathways within the crystal, affecting the material's conductivity and reactivity[103,104]. The unit cell of the rhombohedral phase typically shows significant anisotropy, with varying degrees of elongation or compression along the a, b, and c lattice constants, resulting in lattice distortion in different directions[105,106]. This distortion and increased lattice strain make the rhombohedral spinel oxide more susceptible to structural changes under high-temperature or high-current-density conditions, potentially leading to the formation of local defects[107]. Overall, the crystal structure of rhombohedral spinel oxides, characterized by significant lattice distortion and complex metal-oxygen coordination, exhibits pronounced anisotropy and lower symmetry, offering a large degree of flexibility for tuning the electronic structure and stability, while also presenting stability challenges under extreme conditions[108,109].
2.2.6 Monoclinic phase
Monoclinic spinel oxides belong to the monoclinic crystal system, characterized by low symmetry and significant lattice distortion. In this structure, A-site metal ions are typically situated in octahedral coordination, while B-site metal ions are distributed between tetrahedral and octahedral positions, forming an asymmetric coordination environment that induces considerable internal lattice strain[110,111]. The arrangement of oxygen ions is not symmetric in the monoclinic phase, exhibiting notable displacements and distortions, which further exacerbate the asymmetry of the lattice. The coordination distance between A-site metal ions and oxygen ions typically ranges from 2.3 to 2.5 Å, while the distance between B-site metal ions and oxygen ions is shorter, typically between 2.1 and 2.3 Å[112]. The different coordination environments of the metal ions result in more complex interactions between the A and B site metal ions, affecting the electron density distribution and charge transfer pathways within the crystal[113,114]. The lattice distortion destabilizes the coordination relationship between metal and oxygen, which may lead to the formation of local defects, particularly under high-temperature or high-current density conditions, further impacting the structural stability[115,116]. The unit cell of monoclinic spinel oxides exhibits notable anisotropy, with large differences between the lattice constants a, b, and c, particularly with elongation or compression along the b-axis[117,118]. The irregular arrangement of oxygen ions induces significant directional lattice distortion. Due to its lower symmetry, the crystal exhibits varying stress along different directions, which may lead to structural changes under extreme conditions. Overall, the crystal structure of monoclinic spinel oxides, with its pronounced lattice distortion and complex metal-oxygen coordination, provides significant flexibility for tuning, but also presents higher stability challenges[119,120].
2.2.7 Triclinic phase
In practice, oxide spinels almost never adopt a true triclinic phase because the spinel framework’s inherent topological and geometric constraints are incompatible with such low symmetry[121]. Because the spinel lattice is locked by a densely packed oxygen scaffold, it favors near-orthogonal angles and tolerates only modest shearing, so the three-axis shear required for triclinic symmetry would impose prohibitive metal-oxygen bond strain and AO4/BO6 polyhedral distortion[122]. Therefore, the structure typically reconstructs into a different framework before a stable triclinic spinel can be reached. The spinel structure is based on a close-packed oxygen lattice with translation vectors of similar length and interaxial angles near 90º, so only limited deviations in a,b,c and α,β,γ are tolerated without disrupting close packing[123]. Within this anion scaffold, A- and B-site cations occupy ordered tetrahedral and octahedral voids, forming a three-dimensional network of corner- and edge-sharing AO4 and BO6 polyhedra. In contrast, achieving triclinic symmetry would require all three lattice constants and angles to become inequivalent with strong non-orthogonality, demanding large anisotropic shifts of the oxygen sublattice and severe tilting/distortion of the coordination polyhedra[124]. Such distortion would force unrealistically short or excessively elongated cation–oxygen/cation–cation distances, disrupt the characteristic spinel connectivity, and build elastic strain beyond what the dense oxide lattice can accommodate. From a crystal-chemical perspective, these requirements render a stable triclinic spinel energetically unfavorable: rather than continuously lowering symmetry to a triclinic metric, the lattice is expected to undergo reconstructive transformations into alternative frameworks once distortion exceeds the tolerance of the close-packed anion scaffold and its tetrahedral–octahedral network[125].
2.3 Phase variants of spinel oxides
In addition to the six major crystalline phases, spinel oxides in practical applications may also exhibit various phase variants, which present unique characteristics through modifications such as metal-oxygen coordination, defect regulation, metastable phase transitions (i.e., non-equilibrium transformations that yield kinetically trapped, higher-energy structures), and strain engineering. These four are classic approaches to generating such variants (Figure 3)[126]. In this section, “primary crystal phases” denote space-group-defined bulk polymorphs with distinct long-range symmetry, whereas “phase variants” refer to within-phase structural modulations that do not necessarily form a new bulk phase but can substantially alter the electronic structure and electrocatalytic behavior[127]. The formation of these phase variants leads to changes in the electronic structure and physical properties of the material, making the understanding of their structural evolution mechanisms critical for optimizing electrocatalytic performance[128,129]. The regulation of the coordination environment can influence the electronic structure and catalytic behavior by altering the coordination number and geometry of metal ions[130,131]. Defect regulation, by introducing oxygen vacancies or metal vacancies, can modulate conductivity and reaction activity. Metastable phase transitions enable the transformation of different crystalline phases into lower-energy phases under specific conditions, improving catalytic performance[132]. Finally, strain engineering adjusts the lattice structure via external stresses, tuning the material's electronic and catalytic properties. Understanding these phase variants and their relationship with performance is essential for the rational design of more efficient spinel oxide catalysts[133,134].
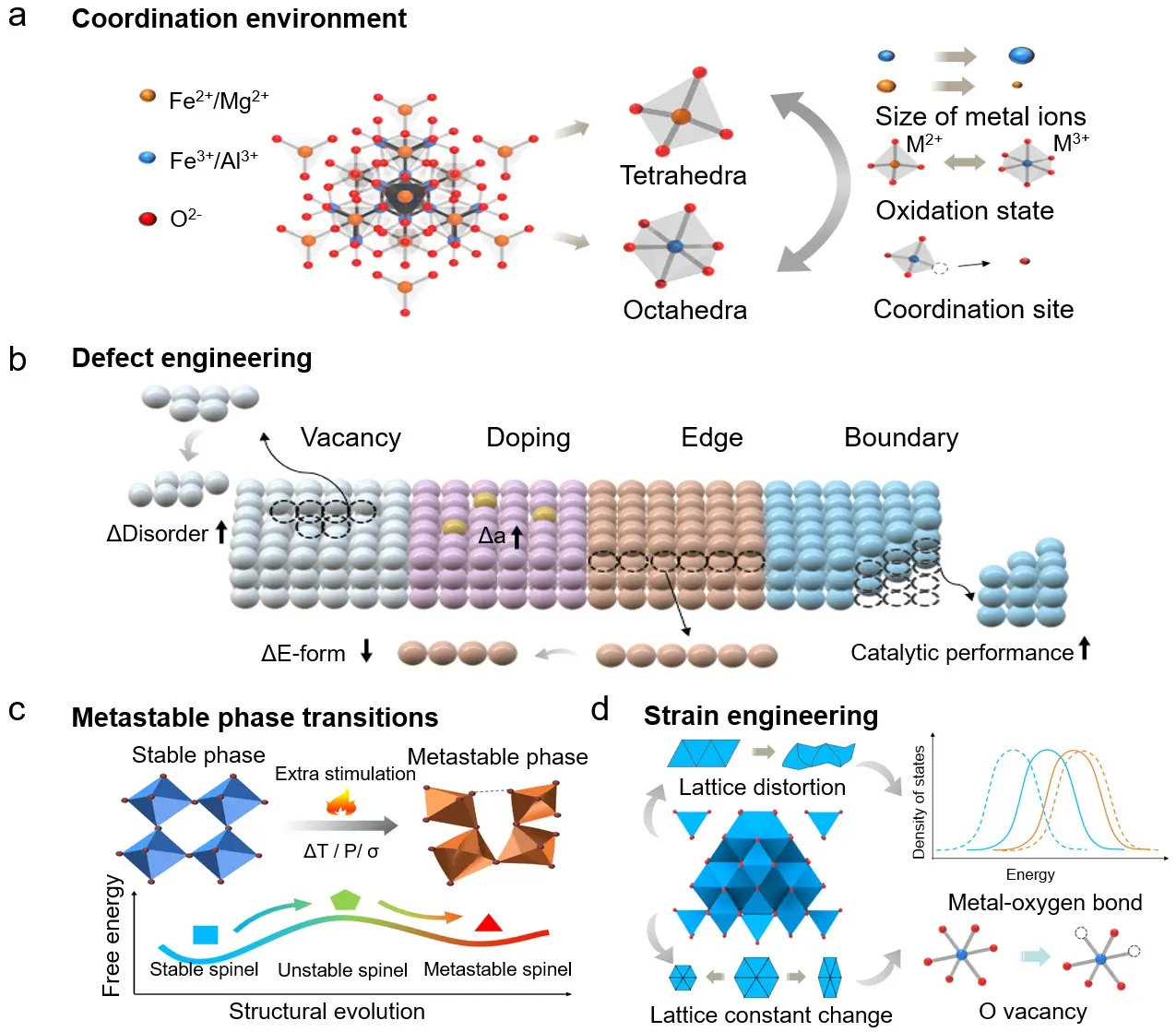
Figure 3. Structural modulation strategies in spinel oxides. (a) Coordination tuning through metal-ion occupation of tetrahedral and octahedral sites, governed by ionic size, oxidation state, and local geometry; (b) Defect engineering, including vacancies, doping, edge and boundary defects, to tailor lattice disorder, formation energy, and reactivity; (c) Metastable phase transitions induced by thermal, chemical, or mechanical stimuli, leading to structural distortion and modified coordination; (d) Strain engineering to adjust lattice constants, metal-oxygen bonding, and electronic states, thereby enhancing catalytic activity.
2.3.1 Coordination environment
The coordination environment of metal ions in spinel oxides plays a pivotal role in determining both the overall structure and properties of the material. In the spinel structure, metal ions typically occupy two types of coordination sites: octahedral and tetrahedral[135]. Metal ions in octahedral sites are coordinated with six oxygen ions, forming an octahedral structure with sixfold coordination, while those in tetrahedral sites are coordinated with four oxygen ions, forming a tetrahedral structure with fourfold coordination[136,137]. The interactions between metal ions in these two coordination environments govern the local geometry of the material and its macroscopic properties. The coordination environment is influenced by factors such as the size, oxidation state of the metal ions, and the presence of vacancies in the lattice[138,139]. For example, smaller metal ions, such as iron and chromium, tend to occupy tetrahedral sites, while larger metal ions, such as cobalt and nickel, are more likely to occupy octahedral sites. Variations in the oxidation states of the metal ions directly affect their electron density, which in turn alters their electronic interactions with oxygen ions[140]. Furthermore, the introduction of coordination vacancies can disrupt the regularity of the coordination environment, leading to changes in the coordination number and geometry of the metal ions, which further impact the electronic structure and catalytic activity. In certain unconventional spinel phases, the coordination environment of metal ions may exhibit asymmetry or irregularity, resulting in incomplete octahedral or tetrahedral coordination[141,142]. Such irregular coordination can enhance the material's reactivity under specific conditions. Therefore, precise control over the coordination environment is crucial for tuning the structural stability and catalytic properties of spinel oxides[143,144]. In conclusion, the coordination environment of metal ions plays a decisive role in regulating the structure and properties of spinel oxides, influencing not only the redox behavior of metal ions but also the overall electronic conductivity and stability of the material[145,146].
2.3.2 Defect regulation
In the structure of spinel oxides, defect regulation is a crucial factor influencing crystal stability and physical properties. The spinel structure consists of alternating metal ions and oxygen ions, with metal ions typically occupying octahedral and tetrahedral sites, while oxygen ions are located at the face centers of the octahedra and the vertices of the tetrahedra[147]. The introduction of defects, particularly oxygen vacancies, metal ion vacancies, and abnormal coordination, causes lattice distortion and generates localized stress, thereby impacting material stability and reactivity. Oxygen vacancies are common point defects in spinel oxides[148,149]. The presence of oxygen vacancies disrupts the periodic arrangement of oxygen ions, resulting in local lattice distortion and stress concentration, which affects the distribution and oxidation states of metal ions[150]. The concentration and distribution of oxygen vacancies determine the stability of metal ions in higher oxidation states, making precise control of oxygen vacancy formation crucial for optimizing material performance. In addition, metal ion vacancies disrupt lattice symmetry, leading to local instability, while abnormal coordination of metal ions further induces disorder in the lattice, affecting the overall structure[151,152]. The introduction of defects is closely linked to lattice strain, particularly as oxygen vacancies, and metal ion vacancies, cause changes in lattice constants, which in turn affect long-range ordering. The presence of defects not only impacts crystal stability but can also induce subtle fluctuations in lattice constants, altering the overall structural symmetry[153,154]. Therefore, precise regulation of defect types, concentrations, and distributions is essential for enhancing the structural stability and catalytic activity of spinel oxides in material design.
2.3.3 Metastable phase transitions
During phase transitions in spinel oxides, metastable phase transitions represent significant phenomena, especially under non-equilibrium conditions where spinel structures may undergo transitions from stable phases to metastable ones[155,156]. These metastable phases typically form under non-equilibrium conditions and possess lower thermodynamic stability, yet they can maintain certain structural characteristics under specific temperature, pressure, or chemical environments[157]. Such phase transitions involve changes in long-range crystalline order and local structural adjustments, with lattice distortions or minor changes in lattice constants often accompanying the formation of metastable phases in metal oxides[158,159]. Structurally, while metastable spinel oxides retain certain aspects of the spinel framework, there are notable changes in crystal symmetry and the coordination environment of metal ions[160]. In these metastable phases, metal ions may deviate from their ideal octahedral or tetrahedral coordination, leading to local stresses and lattice distortions. For instance, reduction or oxidation reactions of metal ions can alter their positions and coordination modes, further influencing the overall structure of the crystal[161]. Moreover, metastable spinel oxides are often associated with significant lattice strain. The formation of metastable phases is usually triggered by factors such as temperature fluctuations, external pressure, or rapid cooling, causing transitions between different phases and resulting in shifts in atomic positions and redistribution of lattice stresses[162]. Although these metastable phases have poorer thermodynamic stability, they can exhibit unique structural features under certain conditions. A deeper understanding of these phase transition processes not only helps elucidate the structural evolution of spinel oxides in varying environments but also provides a theoretical basis for designing materials with specific properties[163,164]. Under realistic OER/HER conditions, metastable spinels may undergo potential-driven surface reconstruction, so the as-prepared phase often serves as a pre-catalyst, while the steady-state reconstructed surface is the true active phase. This should be verified by correlating electrochemical activation/stability with in situ/operando phase/valence tracking (e.g., XAS/Raman/XRD or microscopy). Metastable-phase evolution can be tracked by changes in OER kinetics and interfacial charge transfer[165], as exemplified by the Li-vacancy–induced disordered phase (≈ 430 mV at 10 mA cm-2, 85.6 mV dec-1, C-dl = 0.39 mF cm-2) indicating accelerated charge transfer and enhanced intrinsic OER kinetics[166].
2.3.4 Strain engineering
Strain engineering plays a crucial role in the structural modulation of spinel oxides, particularly in adjusting their crystal characteristics. By applying external stress or introducing strain during the synthesis process, changes can occur in the lattice constants, atomic distances, and the coordination environment of ions in spinel oxides[167,168]. Strain regulation can be achieved via lattice-coupled architectures, where coherent epitaxial interfaces impose a uniform strain field that tunes metal-oxygen bonding and reaction energetics during catalysis[169]. For example, an interfacial lattice-coupling Ru–Mn–O system (Ru, Mn)2O3-250 delivers 168 mV at 10 mA cm-2 with a 68.7 mV dec-1 Tafel slope and sustains > 40 h stability, indicating that interface-induced strain can concurrently enhance charge transfer and structural robustness in line with general strain effects[170]. These strain-induced structural changes are typically manifested as the disruption of crystal symmetry, fine-tuning of lattice constants, and rearrangement of atomic positions. The geometric distortions caused by strain often lead to alterations in metal-oxygen bond strength, which in turn facilitates the formation of oxygen vacancies, creating new active sites[171,172]. Such structural changes often result in a shift in the coordination number of metal ions, for example, from a standard octahedral coordination to a tetrahedral or other asymmetric coordination[173]. These changes in coordination and structure not only affect the crystal’s stability but may also drive new phase transitions, thereby altering the material's overall structural properties. In certain cases, strain can induce a transition in the crystal’s space group, causing a metastable phase to become the dominant phase, thereby imparting new structural characteristics to the material[174,175]. Precise control over strain provides a means of optimizing the structure of spinel oxides. Through strain-guided phase transitions, it is possible to modify the local geometric and electronic states of the crystal without disrupting its overall integrity. The core of strain engineering lies in the fine-tuning of the stress state within the lattice, enabling the optimization of material properties at the microscopic level[176,177]. This structural modulation approach allows for significant performance enhancement while maintaining material stability, particularly in the design of multifunctional catalytic materials.
2.4 Influence of crystal phases on electrocatalytic water splitting mechanisms and performance
The crystal phase structure of spinel oxides significantly influences their electrocatalytic water splitting performance through various mechanisms, including metal ion coordination environment, oxygen vacancy formation, lattice distortion, surface energy, and charge transfer; these phase-dependent structural descriptors collectively regulate active-site chemistry and metal-oxygen interactions, providing a unified framework for the discussion below (Figure 4). Firstly, the coordination environment of metal ions determines the electronic structure, which directly impacts catalytic activity[178]. In cubic spinel oxides, metal ions are typically located in symmetric octahedral coordination, which ensures high stability and efficient electron conductivity, making cubic phases conducive to charge transfer during catalytic reactions. In contrast, low-symmetry phases such as hexagonal and tetragonal phases feature less symmetric and more distorted coordination environments[179,180]. This irregular coordination enhances interactions between metal ions and oxygen intermediates, lowering reaction energy barriers and improving catalytic activity. For example, reduced symmetry and looser metal coordination in these phases allow better interaction between metal ions and oxygen species, accelerating redox reactions[181,182].
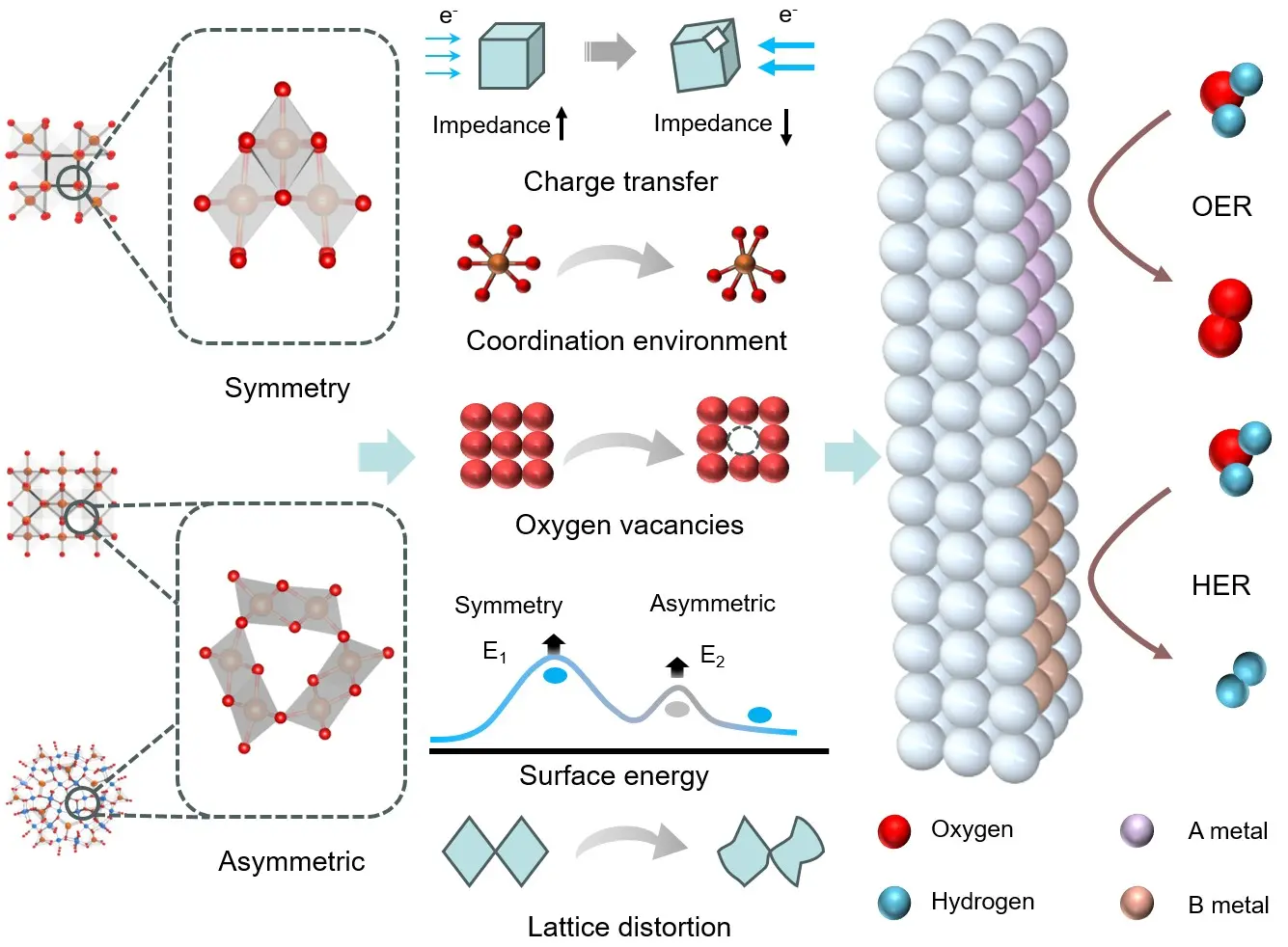
Figure 4. Structural factors regulating water-splitting activity in spinel oxides. Symmetry-dependent coordination environments, oxygen-vacancy formation, lattice distortion, surface-energy variation, and charge-transfer characteristics collectively tune active sites and metal-oxygen interactions, thereby governing OER and HER performance. OER: oxygen evolution reaction; HER: hydrogen evolution reaction.
Secondly, the formation of oxygen vacancies is critical for enhancing catalytic activity in water splitting reactions. Oxygen vacancies act as additional active sites that promote charge transfer and facilitate the reaction[183,184] In high-symmetry structures like the cubic phase, the formation of oxygen vacancies is relatively difficult due to the tight coordination between metal and oxygen ions[185]. However, in lower-symmetry phases such as hexagonal and tetragonal phases, the presence of lattice distortions and asymmetric coordination makes oxygen vacancy formation easier, which effectively lowers reaction barriers, particularly in the OER[186,187]. Oxygen vacancies not only enhance oxygen desorption but also accelerate the formation of reaction intermediates, thereby improving the overall catalytic performance.
Lattice distortion also plays a crucial role in catalysis. This distortion, often caused by irregular metal coordination, oxygen vacancy creation, or external strain, alters the lattice energy distribution, impacting the strength of metal-oxygen bonds and the electron density[188,189]. For instance, in tetragonal and hexagonal phases, the slight changes in the metal-oxygen distance due to lattice distortion strengthen the metal-oxygen bond’s electronic exchange capacity, making metal ions more involved in electron transfer reactions[190]. Lattice distortion may also introduce new catalytic active sites, further enhancing reactivity.
Surface energy is another important factor influencing catalytic efficiency. The surface energy distribution varies between crystal phases, affecting the arrangement of surface active sites. In high-symmetry phases like cubic spinels, surface energy is relatively uniform, and fewer active sites are available, resulting in lower catalytic efficiency[191,192]. However, in lower-symmetry phases like hexagonal and tetragonal phases, lattice distortions and asymmetric coordination lead to more uneven surface energy distribution, creating additional active sites that effectively adsorb and activate reactants, thereby enhancing catalytic activity[193].
Additionally, charge transfer rates are a critical factor in electrocatalytic performance. In lower-symmetry phases, due to lattice irregularities and the presence of oxygen vacancies, the electronic conductivity and charge transfer rates are typically higher, allowing stable catalytic activity even under high current densities[194,195]. Efficient charge transfer in the OER, for example, facilitates the breakdown of water molecules, enhancing the overall reaction rate.
2.5 Summary and statistical analysis of structure–performance relationships in spinel oxide catalysts
The statistical patterns in Table 1 highlight several clear relationships between crystal phases and electrocatalytic performance. We further quantified phase-resolved overpotential–Tafel correlations and phase-wise distributions, noting that protocol heterogeneity may weaken global trends, while phase-dependent shifts remain robust. The phase distribution and metrics in Table 1 are inevitably literature-skewed—cubic spinels are overrepresented because they are thermodynamically favored and readily accessible, whereas low-symmetry phases (e.g., monoclinic) are less frequently and unambiguously reported due to narrower synthesis windows/kinetic stabilization and challenging phase verification under operation; moreover, intrinsic activity descriptors (mass activity, TOF, ECSA-normalized values) are not consistently reported with comparable protocols, so we include/discuss them only when explicitly available and note the resulting limitations for cross-phase comparisons. Cubic spinels, which constitute the largest portion of the dataset, generally exhibit OER overpotentials concentrated near 300 to 360 mV at benchmark current densities in 1.0 M KOH, with Tafel slopes commonly falling in the 50 to 90 mV dec-1 range. Within this phase family, NiCo2O4 and related Co rich compositions repeatedly deliver lower overpotentials, for example values close to 310 mV or 308 mV, while Fe containing cubic systems often occupy the higher end of the range, indicating that B site electronic configuration is a primary contributor to the observed variation. Several cubic entries also display good durability, with operation times such as 20 h at 10 mA cm-2 or stable performance across 1,000 cycles, suggesting that the high symmetry lattice provides consistent structural robustness. In contrast, tetragonal and orthorhombic spinels show a wider but frequently more favorable distribution of activity. For instance, ZnMn2O4 and Mn3O4 exhibit overpotentials near 350 mV or even below 300 mV at comparable current densities, and stability measurements such as 20 h at 10 mA cm-2 or over 300 mA cm-2 conditions imply that lattice distortion or anisotropic bonding can support sustained operation. Hexagonal spinels appear less common in the dataset but show a recognizable pattern of moderate overpotentials, for example values around 370 to 450 mV, combined with strong cycling endurance, often exceeding several hundred cycles. This recurring combination suggests that layered anion and cation arrangements can buffer structural stress during prolonged testing. Monoclinic entries, although fewer, provide activity levels near 270 to 285 mV, accompanied by operation durations such as 12 h at 10 mA cm-2, demonstrating that low symmetry does not necessarily hinder performance when cation distribution is optimized. Across multiple symmetry classes, mixed B site compositions and mild A site doping, such as Li containing Co spinels, appear repeatedly among the better performing entries, with overpotentials near 390 mV or lower and stability values approaching tens of hours, indicating that small perturbations in cation distribution can tune conductivity and intermediate adsorption more effectively than large compositional shifts. Overall, the statistical evidence points to a combined influence of crystallographic symmetry, cation arrangement, and defect related tuning in determining the activity and durability of spinel oxide electrocatalysts, with both high symmetry and symmetry lowered lattices capable of strong performance when supported by optimized electronic and structural environments.
| Crystalline Phases | Material | Electrolyte | Application/Function | Tafel slope (mV dec-1) | Overpotential (mV) | Key structural factor | Stability @Current density | Reference |
| Cubic Spinel Oxides | (R–COO) xσ-Co3O4-x | 1.0 M KOH | OER | 60.9 | η10 = 230 η100 = 330 | Surface functionalization | 100 h @ 20 mA cm-2 | [196] |
| Co2MnO4 | 1.0 M KOH | OER | 65 | η10 = 328 | Mixed-valence Co/Mn | 55 h @ 10 mA cm-2 | [197] | |
| CoMn2O4 | 1.0 M KOH | OER | 92 | η10 = 460 | B-site substitution | 55 h @ 10 mA cm-2 | [197] | |
| Co2MnO4 | H3PO4/H2SO4 | OER | 80 | η500 = 1,700 | Phase robustness under harsh electrolyte | > 1,500 h @ 200 mA cm-2 | [198] | |
| NiCo2O4 | 1.0 M KOH | OER | 58 | η10 = 310 | Mixed-cation conduction network | 100 h @ 10 mA cm-2 | [199] | |
| Co2FeO4 | 1.0 M KOH | OER | 43 | η0.01 = 359 | Fe substitution | 1,000 cycles | [200] | |
| CoFe2O4 | 1.0 M KOH | OER | 79 | η0.01 = 432 | Fe–Co electronic coupling | 1,000 cycles | [200] | |
| Co3O4 | 1.0 M KOH | OER | 65 | η10 = 360 | Baseline cubic framework | 20 h @ 10 mA cm-2 | [201] | |
| Co1.47Rh1.53O4 | 1.0 M KOH | OER | 59.2 | η10 = 301 | Noble-metal substitution | > 24 h @ 10 mA cm-2 | [202] | |
| ZnCo2O4 | 1.0 M KOH | OER | 60 | η10 = 340 | A-site Zn tuning | 1,000 cycles (no η shift) | [201] | |
| CoAl2O4 | 1.0 M KOH | OER | 95 | η10 = 480 | Inactive-site dilution | Stable (low activity) | [201] | |
| Fe1Co1Ox | 1.0 M KOH | OER | 36.8 | η10 = 308 | Fe–Co electronic synergy | > 2.8 h @ 300 mV | [203] | |
| LixCo3-xO4 | 1.0 M KOH | OER | 60-70 | η10 = 390 | Li-induced cation disorder | 60 h @1.62 V | [204] | |
| (Co, Fe, Ni, Cr, Mn)3O4 | 1.0 M KOH | OER | 34.7 | η10 = 307 | High-entropy cation disorder | 168 h @ 10 mA cm-2 | [204] | |
| Mn [Al0.5Mn1.5] O4 | 1.0 M KOH | OER | 60 | η0.025 = 240 | Aliovalent doping | 12 h | [205] | |
| LT-LiCoO2 | 0.1 M KOH | OERORR | 5260 | η10 = 370 | Metastable Li–Co framework | 6 h @1.7 V | [206] | |
| Hexagonal Spinel Oxides | Zn4-xCoxSO4(OH)6·0.5 H₂O | 0.5 M KOH | OER | 60 | η10 = 370 η100 = 450 | Hexagonal open framework | 11 h @380 mV | [207] |
| LiNi0.5Co0.2Mn0.3O2 | 0.1 M KOH | OER | 85.6-241.8 | η10 = 430 | Li-vacancy–induced cation disorder | > 500 cycles | [165] | |
| Co3O4 | 0.05 M KOH | OER | — | — | Hexagonal polymorph | several cycles | [170] | |
| Orthorhombic Spinel Oxides | (Ru, Mn)2O3 | 0.1 M HClO4 | OER | 38 | η10 = 180 | Interface-induced lattice strain | 250 h @ 10 mA cm-2 | [208] |
| Tetragonal Spinel Oxides | Mn3O4 | 0.1 M KOH | OERORR | 8777 | η10 = 410 | Jahn–Teller tetragonal distortion | 2.8 h @1.6 V | [209] |
| CoMn2O4 | —— | OERHER | 8379 | η10 = 252 | Tetragonal distortion | 20 h @ 10 mA cm-2 | [210] | |
| ZnMn2O4 | 1.0 M KOH | OERHER | 84102 | η10 = 350 | Distortion-enabled vacancies | 20 h @ 10 mA cm-2 | [211] | |
| Mn3O4 | 1.0 M KOH | OER | 86 | η10 = 287 | Distortion plus surface reconstruction under high rates | 10 h @ 320 mA cm-2 | [212] | |
| Monoclinic Spinel Oxides | NiCo2Se4 | 1.0 M KOH | OERHER | 63 | η10 = 270 | Anion substitution (Se) | 12 h @ 10 mA cm-2 | [213] |
| NiCo2Se4/Co3Se4 | 1.0 M KOH | OER | 68 | η10 = 285 | Phase boundary | 20 h @ 10 mA cm-2 | [214] |
3. Crystalline Structure-Dependent Electrocatalytic Behavior of Spinel Oxide
The following sections provide a detailed analysis of the electrocatalytic behavior of spinel oxides across different crystal phases, with emphasis on how each structural motif governs performance in electrocatalytic water splitting. Through an in-depth examination of these crystal phases, a clearer understanding can be established regarding the intrinsic connections between crystal structure and catalytic activity, thereby offering a theoretical foundation for the rational optimization of electrocatalysts. To bridge Section 2 and Section 3, we adopt a concise “material–structure–behavior–performance” linkage: composition, phase, and variants define symmetry/coordination, defects/strain and related structural descriptors, which govern intermediate adsorption/charge transport and possible reconstruction under operating potentials, ultimately determining activity–stability outcomes across the phase-by-phase discussion below.
3.1 Cubic spinel oxides
Following the previous discussion on phase-governed activity, Cheng et al. established a rapid room-temperature reduction-recrystallization strategy to synthesize nanocrystalline Co-Mn-O spinels with precisely controllable cubic and tetragonal phases[215]. As illustrated in Figure 5a, the cubic CoMn2O4 lattice consists of corner-sharing [Co/MnO6] octahedra and [Co/MnO4] tetrahedra, forming a highly symmetric three-dimensional framework that enables efficient electron transport. First-principles simulations indicate that the cubic (113) surface exhibits a stronger oxygen adsorption energy (Eb = -2.42 eV), and an Oads-induced d-band upshift toward the Fermi level, revealing intensified metal-oxygen orbital hybridization (i.e., covalent mixing/overlap between transition-metal 3d and O 2p states) compared with the tetragonal (121) surface (Figure 5b,c). Electrochemical characterization further demonstrates that the cubic phase delivers superior oxygen-reduction activity, whereas the tetragonal counterpart favors enhanced oxygen-evolution performance, both outperforming conventionally synthesized high-temperature spinels (Figure 5d). These findings clearly demonstrate that crystallographic symmetry and defect-mediated electronic modulation cooperatively regulate charge transport and intermediate adsorption, underscoring that phase engineering of spinel oxides provides an effective strategy to optimize bifunctional electrocatalysis for overall water splitting. Extending the investigation on Co-Mn-O spinels, Li et al. employed a controlled thermal-decomposition strategy to prepare cubic Co2MnO4 spinel catalysts with enhanced structural stability and acid durability[198]. The catalyst adopts a cubic Fd-3m lattice in which Co2+ occupies tetrahedral sites while Mn3+/4+ resides at octahedral positions (Figure 5e). Charge redistribution across Co-O-Mn coordination units builds a rigid oxygen network that effectively suppresses lattice distortion and prevents structural collapse during oxygen evolution (Figure 5f). First-principles calculations reveal that the dominant (311) surface exhibits near-optimal adsorption energies for OER intermediates (Ead (OH*) ≈ -0.8 eV; Ead (O*) ≈ -0.3 eV), ensuring balanced binding strength and mitigating metal dissolution under acidic conditions (Figure 5g). Electrochemical measurements confirm remarkable activity, showing an overpotential of 0.25 ± 0.01 V at 500 mA cm-2 and stable operation exceeding 1,500 h in pH 1 electrolyte (Figure 5h). These results demonstrate that cation ordering and Mn-mediated Co-O stabilization cooperatively modulate the electronic structure and surface energetics, highlighting that precise crystallographic regulation in cubic spinels can simultaneously achieve high activity and exceptional acid stability for overall water-splitting electrocatalysis.
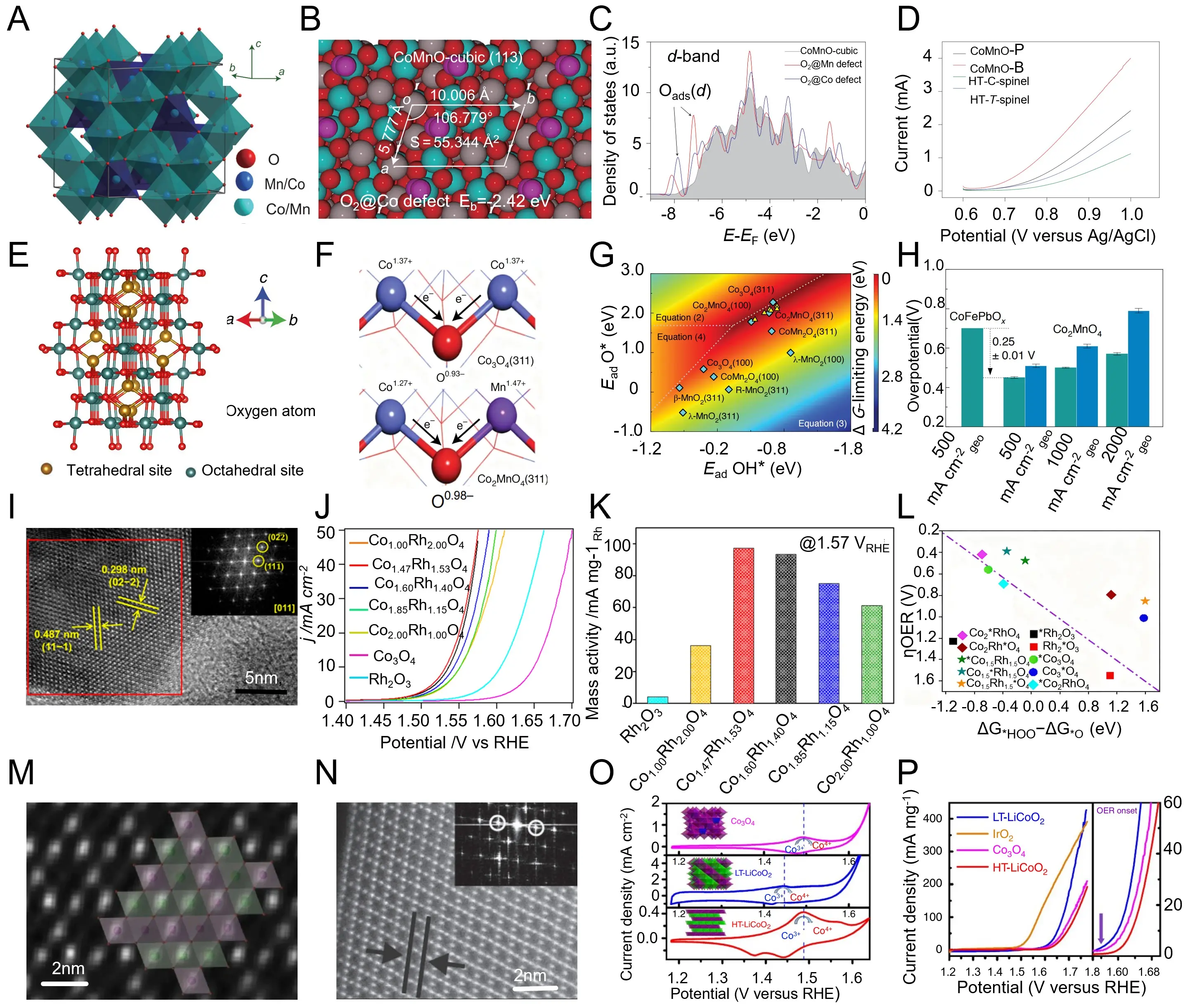
Figure 5. (a) Crystal structure model of Co-Mn-O spinel showing Mn/Co octahedra; (b) Atomic configuration and oxygen-vacancy formation energy in Co-Mn-O with highlighted local structural parameters; (c) Calculated DOS for Co-Mn-O showing d-band modulation induced by oxygen-vacancy configurations; (d) Electrochemical polarization curves of various Co–Mn–O phases (CoMnO-P, CoMnO-B, HT-C-spinel, HT-T-spinel). Republished with permission from[215]; (e) Crystal structure model of Co2MnO4 showing tetrahedral and octahedral cation sites; (f) Schematic of surface adsorption configurations (OH-, O) on Co2MnO4(311) and Co3O4(311) surfaces; (g) Energy landscape diagram correlating Ead(O) and E3d(O) for different Co-Mn-O surfaces; (h) Comparison of OER overpotentials of Co2MnO4 and Co-Fe-B2O4 under different current densities. Republished with permission from[198,216]; (i) HRTEM image and FFT pattern of CoxRh3-xO4 showing well-ordered spinel lattice; (j) LSV polarization curves of CoxRh3-xO4 spinels showing composition-dependent activity; (k) Mass activity comparison of RhO2, Co3O4, and CoxRh3-xO4 at 1.57 V RHE; (l) Scaling relationship between log(OER activity) and ΔGHOO - ΔGO for various CoxRh3-xO4 compositions. Republished with permission from[202]; (m) Atomic-resolution HAADF-STEM image of LT-LiCoO2 showing the local spinel-like atomic arrangement; (n) HRTEM image and FFT pattern showing lattice ordering and phase transition features in LiCoO2; (o) LSV polarization curves comparing Co3O4, HT-LiCoO2 and LT-LiCoO2; (p) OER polarization and ORR activity comparison of LiCoO2-derived materials and reference Co3O4. Republished with permission from[206]. DOS: density of states; OER: oxygen evolution reaction; HT: high temperature; LT: low temperature; FFT: fast Fourier transform; ORR: oxygen reduction reaction; LSV: linear-sweep voltammograms; RHE: reversible hydrogen electrode; HRTEM: high-resolution transmission electron microscopy; HAADF-STEM: high-angle annular dark-field scanning transmission electron microscopy; C-spinel: cubic spinel; T-spinel: tetragonal spinel.
To further elucidate the role of cation modulation in cubic spinels, Zhu et al. constructed a series of CoxRh3-xO4 solid-solution spinels via high-temperature solid-state synthesis, enabling precise control of lattice distortion and electronic configuration through Rh incorporation[202]. As shown in Figure 5i, the catalysts retain a well-defined Fd-3m cubic framework, with high-resolution TEM images revealing distinct (111) and (022) planes (0.487 and 0.298 nm, respectively), confirming homogeneous Co/Rh distribution. Electrochemical polarization curves and mass-activity plots demonstrate that the optimized Co1.85Rh1.15O4 exhibits an overpotential of 270 mV at 10 mA cm-2 and an exceptional mass activity of ≈ 90 mA mg-1Rh @ 1.57 V vs reversible hydrogen electrode (RHE), outperforming Co3O4 and Rh2O3 benchmarks (Figure 5j,k). Theoretical calculations further reveal a volcano-type relationship between overpotential and adsorption-energy difference (ΔG*OOH - ΔG*O), indicating optimized intermediate binding arising from lattice distortion and Co-Rh electronic hybridization (Figure 5l). Collectively, these findings demonstrate that rational lattice engineering and cation substitution within cubic spinels effectively tailor orbital occupancy and reaction thermodynamics, thereby substantially accelerating intrinsic oxygen-evolution kinetics. Building on the preceding advances in cation-engineered spinels, Hong et al. synthesized low-temperature (LT) and high-temperature (HT) LiCoO2 phases through a controlled solid-state reaction, allowing a direct comparison between cubic-like disordered and layered rhombohedral structures[206]. As shown in Figure 5m,n, high-resolution STEM images reveal that LT-LiCoO2 exhibits partially disordered Co-O stacking resembling a spinel-like cubic configuration, while HT-LiCoO2 maintains a well-ordered layered framework with distinct (003) lattice fringes. Such structural divergence markedly alters the electronic configuration and surface coordination, leading to distinct oxygen-adsorption behaviors. Electrochemical measurements demonstrate that LT-LiCoO2 achieves superior oxygen-evolution activity with an overpotential of ≈ 350 mV at 10 mA cm-2, outperforming both HT-LiCoO2 and Co3O4 references (Figure 5o,p). These findings confirm that phase transformation from layered to spinel-like cubic structures enhances active-site accessibility and charge transport, emphasizing that crystal-phase reconstruction is a key pathway to boost the intrinsic OER activity of cobalt-based oxides.
3.2 Hexagonal spinel oxides
Following the previous discussion on phase-regulated Co-based systems, Huang et al. synthesized LiNi0.5Co0.2Mn0.3O2 (NCM523) with layered (HT-NCM), spinel-like (LT-NCM), and disordered (DO-NCM) phases via a polymer-assisted solution synthesis, aiming to correlate lithium-vacancy formation with oxygen-evolution activity[165]. As illustrated in Figure 6a, schematic analysis depicts that Li deficiency and cation intermixing disrupt the regular stacking of the layered structure, leading to progressive lattice disorder. Figure 6b shows the XRD patterns, where the gradual reduction of the (003)/(104) intensity ratio confirms the transformation from the well-ordered R-3m layered lattice to a disordered hexagonal configuration. Electrochemical measurements reveal that the DO-NCM phase exhibits the highest catalytic activity, with an overpotential of ≈ 430 mV @ 10 mA cm-2 and a Tafel slope of 85.6 mV dec-1, surpassing both LT- and HT-NCM counterparts (Figure 6c). In addition, capacitive response analyses show that DO-NCM possesses the largest double-layer capacitance (0.39 mF cm-2), indicating increased electrochemically active surface area and faster charge transfer (Figure 6d). These results demonstrate that Li-vacancy-induced cation disorder and crystal-phase transformation synergistically enhance electronic delocalization and active-site accessibility, underscoring that phase modulation is a decisive factor governing the intrinsic OER kinetics of layered transition-metal oxides. Building on the preceding insights into phase- and structure-regulated layered oxides, Varhade et al. employed scanning electrochemical cell microscopy (SECCM) to elucidate the facet-dependent electrocatalytic behavior of single hexagonal Co3O4 spinel crystals[170]. As shown in Figure 6e, high-resolution scanning electron microscopy (SEM) images identify individual microcrystals with well-defined (111) top and (110) edge facets, characteristic of the hexagonal spinel lattice. AFM analyses further reveal distinct surface morphologies and facet thicknesses, confirming anisotropic Co-O coordination and crystallographic orientation (Figure 6f). Electrochemical mapping demonstrates that the (111) facets exhibit a thickness-dependent turnover frequency (TOF) of approximately 100 s-1 at 1.8 V vs RHE, whereas the (110) planes achieve a higher and thickness-independent TOF of ≈ 270 ± 68 s-1 (Figure 6g). The corresponding TOF-thickness correlation indicates that enhanced surface Co density and improved electron conductivity along the (110) edges account for the superior oxygen-evolution performance (Figure 6h). These results highlight that crystal-plane anisotropy plays a decisive role in dictating local reaction kinetics, underscoring that facet-level structural regulation is an effective route to optimize the intrinsic activity of hexagonal spinel oxides.
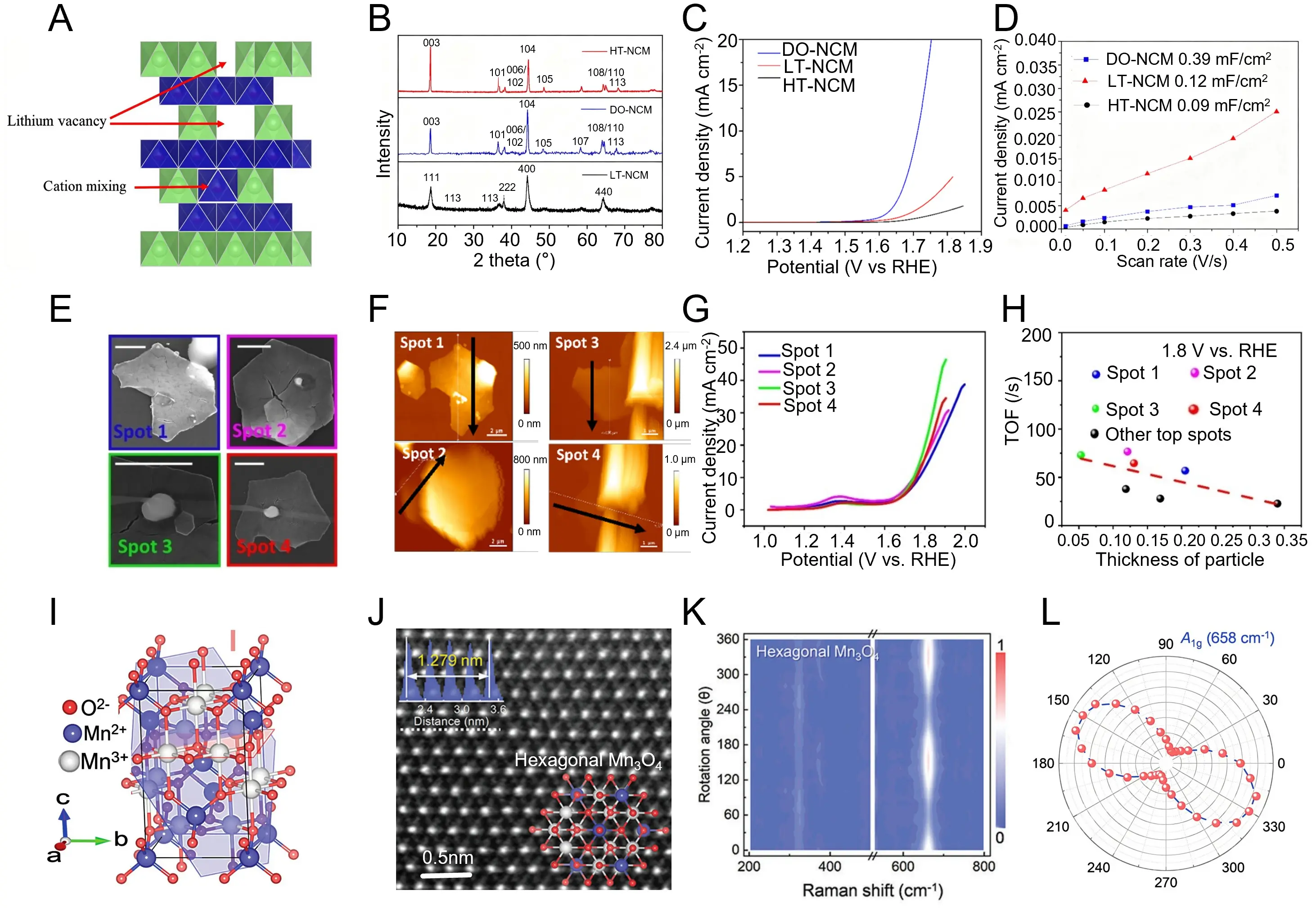
Figure 6. (a) Schematic illustration of cation mixing and lithium-vacancy defects in layered NCM structures; (b) XRD patterns of NCM samples showing the structural evolution associated with heat treatment and cation disorder; (c) LSV of LT-, HT-, and DO-NCM catalysts in 0.1 M KOH; (d) Scan-rate-dependent current responses of LT-, HT-, and DO-NCM electrodes, indicating different capacitive behaviors. Republished with permission from[165]; (e) SEM image of a single hexagonal Co3O4 particle analyzed by SECCM; (f) Cyclic-voltammogram responses at landing spots on particle surfaces and bare carbon; (g) SEM micrograph showing on-top droplet landing spots on the Co3O4 particle used for activity mapping; (h) Corresponding TOF values of the (111) top plane plotted against particle thickness. Republished with permission from[170]; (i) Schematic of the CVD growth of 2D tetragonal and hexagonal Mn3O4 nanosheets; (j) High-resolution TEM images revealing lattice arrangements and thickness of tetragonal and hexagonal Mn3O4 nanosheets; (k) Angle-dependent Raman spectra of hexagonal Mn3O4 showing crystallographic anisotropy; (l) Polar plot of the A1g Raman mode (658 cm-1) confirming the single-crystal symmetry of the Mn3O4 nanosheets. Republished with permission from[166]. LSV: linear-sweep voltammogram; HT: high temperature; LT: low temperature; SEM: scanning electron microscopy; TEM: transmission electron microscopy; SECCM: scanning electrochemical cell microscopy; XRD: X-ray diffraction; LSV: linear sweep voltammetry; TOF: turnover frequency; NCM: nickel-cobalt-manganese; DO: disordered oxide; CVD: chemical vapor deposition.
Following the above study on facet-controlled Co3O4 systems, Feng et al. achieved the phase-engineered synthesis of two-dimensional hexagonal Mn3O4 spinel nanosheets via a van der Waals epitaxial growth strategy, enabling precise control over crystal geometry and orientation[166]. Atomic-resolution structural modeling reveals a well-defined hexagonal spinel lattice composed of alternating Mn2+ and Mn3+ ions within a layered framework (Figure 6i). High-resolution STEM imaging further displays periodically ordered atomic planes with a lattice spacing of ~0.279 nm, confirming the formation of single-phase hexagonal Mn3O4 (Figure 6j). Raman spectroscopy identifies a strong A1g vibration at 658 cm-1 with pronounced angular dependence, indicative of high crystallinity and in-plane symmetry (Figure 6k,l). Compared with conventional tetragonal Mn3O4, the hexagonal phase exhibits enhanced electronic coupling and faster charge transfer, arising from improved metal-oxygen orbital hybridization. These findings highlight that the tetragonal-to-hexagonal phase transformation effectively modulates the electronic configuration and coordination environment, demonstrating the pivotal role of phase engineering in optimizing the catalytic performance of Mn-based spinel oxides.
3.3 Orthorhombic spinel oxides
Following the above discussion on oxide-phase engineering, Liu et al. constructed a layered Ru-Mn-O mixed oxide through an interfacial lattice-coupling strategy, enabling atomic-level modulation of metal-oxygen coordination and crystallographic coherence[208]. Structural modeling reveals a layered configuration with an interplanar spacing of 8.99 Å, where alternately arranged RuO6 and MnO6 octahedra form coherent heterointerfaces between RuO2 and Mn2O3 domains (Figure 7a). High-resolution TEM imaging displays lattice fringes of 0.247 nm corresponding to Ru-Mn-O and 0.211 nm for Mn2O3, confirming epitaxial coupling and structural continuity across the phase boundary (Figure 7b). Fast Fourier transform (FFT) pattern analysis further corroborates this coherent interface alignment, indicating ordered orientation and uniform lattice strain (Figure 7c). Electrochemical evaluation shows that the optimized (Ru, Mn)2O3-250 catalyst achieves an overpotential of 168 mV @ 10 mA cm-2 and a Tafel slope of 68.7 mV dec-1, maintaining stability for over 40 h (Figure 7d). These results highlight that lattice coupling and phase hybridization effectively tailor the electronic structure and reaction energetics, providing a viable strategy to enhance the intrinsic OER activity of Ru-based mixed oxides. Expanding the exploration of crystallographic modulation in Mn-based systems, Du et al. synthesized orthorhombic post-spinel CaMn2O4 nanorods through a facile solvothermal method, achieving controllable phase formation under moderate conditions[217]. Structural modeling reveals a marokite-type orthorhombic framework composed of distorted MnO6 octahedra and CaO8 polyhedral that assemble into a robust three-dimensional lattice (Figure 7e). High-resolution TEM analysis shows clear (023) lattice fringes with a spacing of 0.273 nm, confirming high crystallinity and preferential orientation along the nanorod axis (Figure 7f). Electrochemical oxygen reduction reaction (ORR) measurements reveal a distinct oxygen-reduction peak around -0.15 V vs SCE for CaMn2O4/C, comparable to Pt/C and indicative of a quasi-four-electron process (Figure 7g). The Koutecký-Levich analysis confirms an electron-transfer number close to 4 with low diffusion resistance, demonstrating efficient oxygen-reduction kinetics (Figure 7h). These findings demonstrate that the orthorhombic post-spinel lattice, stabilized by Ca-Mn coordination and enhanced electron transport, provides an effective route to optimize the intrinsic ORR activity of Mn-based spinel oxides.
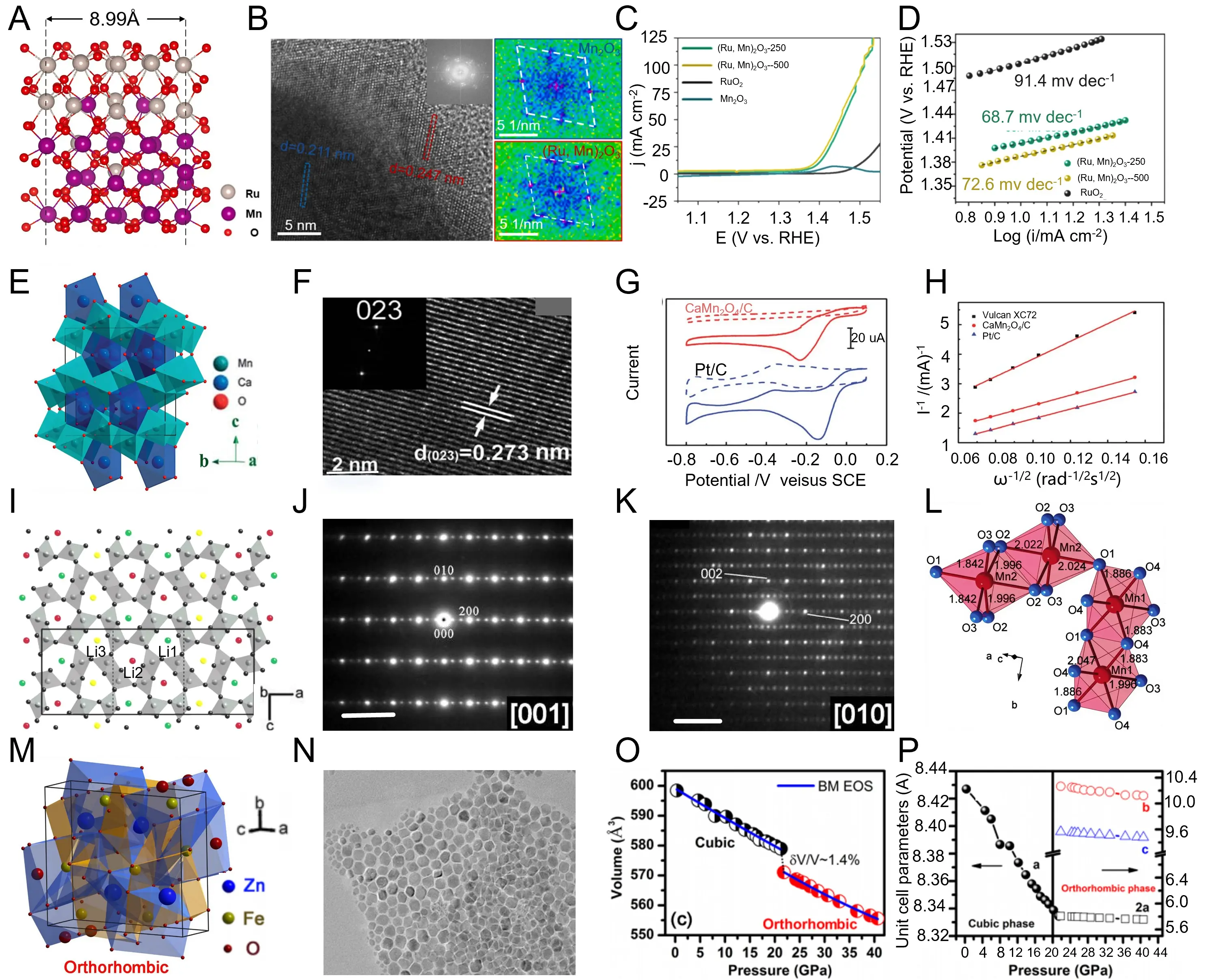
Figure 7. (a) Schematic illustration of the orthorhombic (Ru, Mn)2O3 crystal structure showing atomic positions of Ru, Mn, and O; (b) High-resolution TEM image and corresponding FFT patterns revealing lattice expansion and strain distribution in (Ru, Mn)2O3; (c) LSVs of (Ru, Mn)2O3 and RuO2 in 0.5 M H2SO4 comparing OER activities; (d) Tafel plots derived from LSV curves showing improved OER kinetics after Ru incorporation. Republished with permission from[208]; (e) Crystal model of orthorhombic CaMn2O4 illustrating MnO6 octahedral connectivity; (f) High-resolution TEM image showing lattice fringes of the (023) plane in CaMn2O4 nanorods; (g) Cyclic voltammograms of CaMn2O4 compared with Pt/C and Vulcan under identical conditions (h) Koutecky–Levich plots obtained from rotating-disk electrode measurements of CaMn2O4 in 0.1 M KOH. Republished with permission from[217]; (i) Crystal structure of orthorhombic LiMn2O4 derived from high-pressure transformation showing Mn-O network; (j) SAED pattern recorded along the [001] zone axis of CaFe2O4-type LiMn2O4; (k) SAED pattern acquired along the [010] direction showing the orthorhombic symmetry of LiMn2O4 (l) Polyhedral representation of orthorhombic LiMn2O4 showing MnO6 connectivity and crystallographic axes. Republished with permission from[218]; (m) Polyhedral structure of orthorhombic ZnFe2O4 highlighting FeO6 and ZnO6 coordination environments; (n) TEM image of ZnFe2O4 nanoparticles showing morphology and particle distribution; (o) Pressure-dependent volume change of ZnFe2O4 showing a cubic-to-orthorhombic transition fitted by a Birch–Murnaghan equation of state; (p) Evolution of lattice parameters with pressure, confirming cubic-to-orthorhombic transition near 20-25 GPa. Republished with permission from[219]. TEM: transmission electron microscopy; LSV: linear-sweep voltammogram; FFT: fast Fourier transform; OER: oxygen evolution reaction; SAED: selected-area electron diffraction.
Advancing from the study of orthorhombic post-spinel frameworks, Yamaura et al. realized a spinel-to-CaFe2O4-type phase transformation in LiMn2O4 using a high-pressure solid-state reaction (6 GPa, > 1,100 °C), achieving denser lattice packing and altered Mn coordination[218]. The transformed CaFe2O4-type structure exhibits orthorhombic pnma symmetry, composed of edge-shared MnO6 octahedral chains with Li ions occupying interstitial sites along the b-axis (Figure 7i). Electron-diffraction patterns display sharp [001] and [010] zone-axis reflections, confirming a 3a × b × c superstructure associated with ordered Li-vacancy distribution (Figure 7j). High-resolution diffraction mapping further supports coherent phase formation and crystallographic ordering within the reconstructed lattice (Figure 7k). Local structural analysis identifies two distinct Mn coordination environments, with Mn1-O distances of approximately 1.89 Å and Mn2-O distances of roughly 1.99 Å, accompanied by a moderate octahedral distortion of about 9%, consistent with a mixed Mn3+/Mn4+ valence configuration (Figure 7l). The high-pressure-induced reconstruction increases structural density by ~6% and decreases the activation energy from 0.40 to 0.27 eV, reflecting improved electron mobility and enhanced stability. Collectively, these findings demonstrate that pressure-driven orthorhombic transformation of spinel lattices provides an effective route to regulate cation ordering and charge transport, deepening the understanding of structural-phase control in Mn-oxide electrocatalysts. Continuing the investigation of pressure-induced lattice reconstruction, Zhang et al. explored the structural evolution of spinel ZnFe2O4 nanoparticles under compression up to 40 GPa using in-situ synchrotron X-ray diffraction[219]. The material initially exhibits a cubic Fd-3m spinel framework composed of tetrahedral Zn2+ and octahedral Fe3+ sites, which progressively transforms into a denser orthorhombic post-spinel phase with mixed Fe-O coordination (Figure 7m). TEM imaging reveals uniform nanocrystals (~10 nm) whose morphology remains intact throughout compression, ensuring homogeneous strain distribution and structural integrity (Figure 7n). Equation-of-state analysis indicates a discontinuous volume collapse of ≈ 1.4% at ≈ 22-25 GPa, marking the onset of the cubic-to-orthorhombic transition (Figure 7o). The evolution of lattice parameters confirms anisotropic compression dominated along the b-axis, consistent with symmetry reduction and rearranged cation coordination (Figure 7p). The high-pressure orthorhombic phase exhibits enhanced electronic density and improved dielectric stability due to reduced grain-boundary resistance and increased Fe-O overlap. These findings demonstrate that pressure-driven symmetry reduction in spinel ferrites effectively modulates electron transport and polarization behavior, highlighting the pivotal role of phase compression engineering in tuning the functional properties of Fe-based spinel oxides.
3.4 Tetragonal spinel oxides
Extending the discussion on structural modulation of spinel frameworks, Zhang et al. realized the in-situ growth of tetragonal CoMn2O4 (CMO-U@CC) arrays on conductive carbon cloth through a controlled hydrothermal-annealing process[210]. As shown in Figure 8a, high-resolution TEM reveals distinct lattice fringes corresponding to the (117) and (011) planes, verifying the well-defined tetragonal spinel structure with coherent interfacial contact. Electrocatalytic analyses demonstrate outstanding bifunctional activity, with overpotentials of 252 mV (OER) and 194 mV (HER) at 10 mA cm-2, surpassing cubic and amorphous counterparts (Figure 8b,c). The integrated electrolyzer achieves overall water splitting at 1.61 V to deliver 10 mA cm-2, retaining performance over 20 h of continuous operation. Such enhancement originates from the tetragonal-induced lattice distortion that strengthens Co/Mn-O hybridization and accelerates surface redox transitions (Figure 8d). These results highlight that tetragonal-phase regulation within Co-Mn-oxide spinels provides a rational route to harmonize conductivity, stability, and bifunctional catalytic efficiency for overall water splitting. Building upon the preceding exploration of tetragonal spinel modulation, Wang et al. prepared ZnMn2O4 nanocrystals via a solvothermal synthesis route, enabling precise control over cation ordering and lattice symmetry[211]. As depicted in Figure 8e,f, the obtained material crystallizes in a single‐phase spinel framework where Zn2+ occupies tetrahedral sites and Mn3+ resides in octahedral sites, as confirmed by distinct SAED rings indexed to the (211), (220), and (428) planes. Electrocatalytic evaluation shows that the optimized ZnMn2O4 delivers an overpotential of ≈ 380 mV at 10 mA cm-2 with a Tafel slope of 148 mV dec-1 for the OER (Figure 8g). The corresponding HER polarization curves display negligible degradation after repeated cycling, highlighting its robust bifunctional stability (Figure 8h). Such superior performance arises from the cooperative effect of Mn-O orbital hybridization and the symmetric spinel geometry, which collectively enhance charge transfer and surface redox kinetics. These results underscore that cation-site distribution and spinel symmetry regulation are decisive factors governing the intrinsic activity and durability of Mn-based spinel oxides.
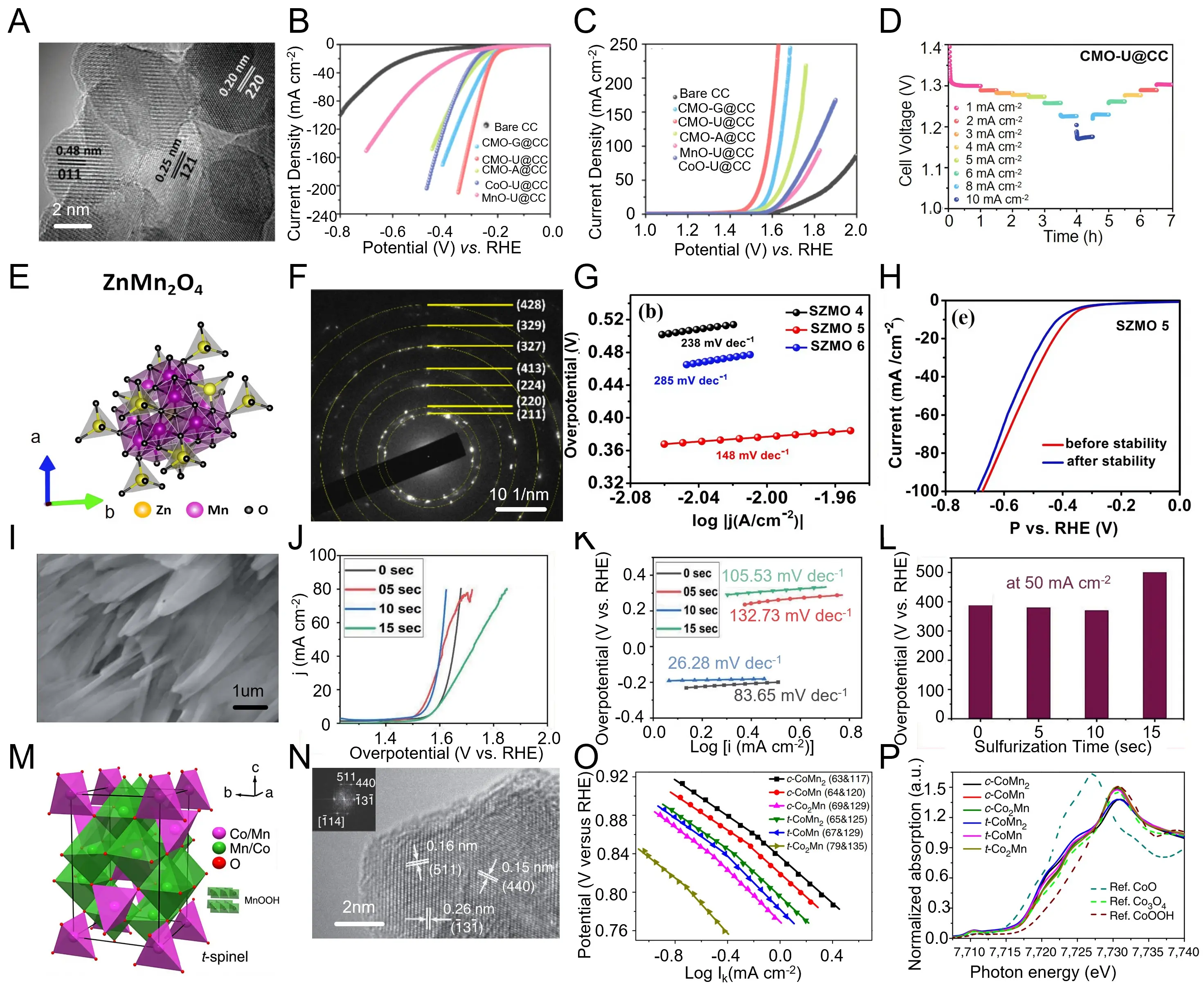
Figure 8. (a) High-resolution TEM image of CoMn2O4 showing lattice fringes assigned to the (011) and (200) planes; (b) LSVs of different Co–Mn oxide/CC electrodes in 1.0 M KOH; (c) Polarization curves of Co–Mn oxide electrocatalysts recorded in 1.0 M KOH; (d) Chronopotentiometry profile of CMO-U@CC at different current densities. Republished with permission from[210]; (e) Crystal model of tetragonal ZnMn2O4 showing Zn and Mn coordination within the spinel lattice; (f) SAED pattern of ZnMn2O4 with indexed diffraction rings; (g) Tafel plots derived from OER polarization curves of SZMO samples showing different Tafel slopes; (h) Linear-sweep voltammograms of SZMO before and after stability testing. Republished with permission from[211]; (i) SEM image showing nanosheet-assembled SZMO morphology; (j) Tafel plots of SZMO electrodes after different sulfurization times; (k) Tafel slope values extracted from polarization curves under different sulfurization durations; (l) Overpotential values of SZMO electrodes at 50 mA cm-2 after selected sulfurization times. Republished with permission from[220]; (m) Crystal model of t-spinel Co/Mn oxides showing octahedral coordination; (n) High-resolution TEM image showing lattice spacings of cubic and tetragonal Co–Mn oxide nanoparticles; (o) Tafel plots comparing electrocatalytic activity of cubic and tetragonal Co–Mn oxide phases; (p) X-ray absorption spectra at the Co K-edge revealing electronic structure differences between cubic and tetragonal Co–Mn oxides. Republished with permission from[209]. LSV: linear-sweep voltammogram; SAED: selected-area electron diffraction; OER: oxygen evolution reaction; TEM: transmission electron microscopy; SEM: scanning electron microscopy.
To further advance the understanding of anion-regulated spinel systems, Bahadur et al. fabricated surface-sulfurized CoMn2O4 spike-like microstructures arrays via a chloride-bath electrodeposition followed by time-controlled sulfurization, enabling precise surface modification while preserving the intrinsic tetragonal spinel framework[220]. As illustrated in Figure 8i, the resulting spike-like microstructures consist of vertically aligned nanosheets that expose abundant edge sites and provide open channels for mass transport. Electrocatalytic analyses reveal that the optimally sulfurized sample (10 s) achieves an overpotential of ≈ 300 mV @ 10 mA cm-2 and a remarkably low Tafel slope of 26 mV dec-1, whereas excessive sulfurization slightly decreases activity due to partial surface passivation (Figure 8j,k). The corresponding stability evaluation confirms robust OER performance at 50 mA cm-2 with negligible degradation over extended operation (Figure 8l). The enhanced catalytic behavior arises from sulfur-induced modulation of Co/Mn oxidation states and the generation of oxygen-vacancy-rich surface sites, which synergistically accelerate charge transfer and intermediate adsorption. These findings demonstrate that controlled surface sulfurization, coupled with preserved spinel symmetry, provides an effective strategy to balance activity and durability in Co-Mn-based oxide electrocatalysts. Following the investigation of sulfurized Co-Mn spinels, Li et al. achieved phase- and composition-controlled synthesis of Co-Mn spinel nanoparticles through a solution-mediated oxidation-precipitation approach, enabling precise regulation between cubic (Fd-3m) and tetragonal (I41/amd) frameworks[209]. As depicted in Figure 8m,n, both structures exhibit highly ordered atomic arrangements with interplanar spacings of 0.15-0.26 nm, reflecting coherent cation distribution within the spinel lattice. Electrocatalytic evaluation demonstrates that the cubic CoMn2O4 phase delivers the lowest overpotential and smallest Tafel slope, while the tetragonal counterpart exhibits slightly reduced activity due to stronger Jahn-Teller distortion (i.e., an electronically driven elongation/compression of MO6 octahedra that lowers local symmetry) and diminished Mn4+ content (Figure 8o). Spectroscopic characterization further confirms that the cubic lattice features higher Mn valence and stronger Co-O hybridization, which collectively promotes charge transfer and oxygen adsorption (Figure 8p). This structural-electronic interplay endows the cubic phase with bifunctional activity comparable to Pt/C and excellent operational stability. Overall, these results underscore that tailoring crystal symmetry and cation valence states is a powerful strategy to optimize the intrinsic electrocatalytic behavior of Co-Mn spinel oxides.
3.5 Other phase structures
Extending the above discussion on structurally modulated spinel systems, Talanov et al. conducted comprehensive crystallographic and electronic-structure analyses to elucidate the rhombohedral transformation of AlV2O4, a geometrically frustrated spinel oxide exhibiting orbital-driven charge ordering[209]. As shown in Figure 9a, the distorted rhombohedral (R-3m) lattice originates from cooperative displacements of Al, V, and O atoms, giving rise to distinct V1, V2, and V3 sites arranged within a Kagome-layered framework. Electron-density mapping reveals localized charge accumulation along V-V linkages, evidencing the formation of clustered V-heptamer or trimer subunits that break the metallic connectivity of the parent cubic phase (Figure 9b,c). The bond-electron profiles further indicate strong covalent interactions across V-O-V bridges, confirming orbital polarization and charge localization within the t2g manifold (Figure 9d). This cooperative lattice distortion substantially modifies the electronic configuration and enhances orbital anisotropy, thereby influencing redox activity and interfacial charge transfer. Overall, the study highlights that rhombohedral distortion and orbital ordering in spinel frameworks can effectively modulate electronic delocalization and catalytic behavior, providing structural insights relevant to phase-engineered spinel oxides. Continuing the exploration of anion-regulated spinel systems, Jeghan et al. synthesized a series of one-dimensional NiCo2O4, NiCo2S4, and NiCo2Se4 nanowires through a hydrothermal route followed by sequential sulfidation and selenization[213]. As shown in Figure 9e, f, the samples display uniform porous nanowire morphologies with high crystallinity, where NiCo2O4 and NiCo2S4 adopt cubic spinel lattices, while NiCo2Se4 transforms into a monoclinic phase upon selenization. This anion substitution induces lattice distortion and electronic delocalization, resulting in enhanced conductivity and abundant surface-active sites. Electrochemical evaluations reveal that NiCo2Se4 achieves an exceptionally low overpotential of 270 mV @ 10 mA cm-2 and a Tafel slope of 63 mV dec-1, maintaining robust stability over 12 h of operation. Such performance surpasses its oxide and sulfide counterparts, highlighting the critical role of anion-induced phase modulation in optimizing charge transfer and active-site accessibility (Figure 9g,h). Overall, this study demonstrates that controlled O→S→Se substitution within spinel frameworks can strategically tune electronic structure and accelerate the oxygen-evolution kinetics in mixed-metal systems.
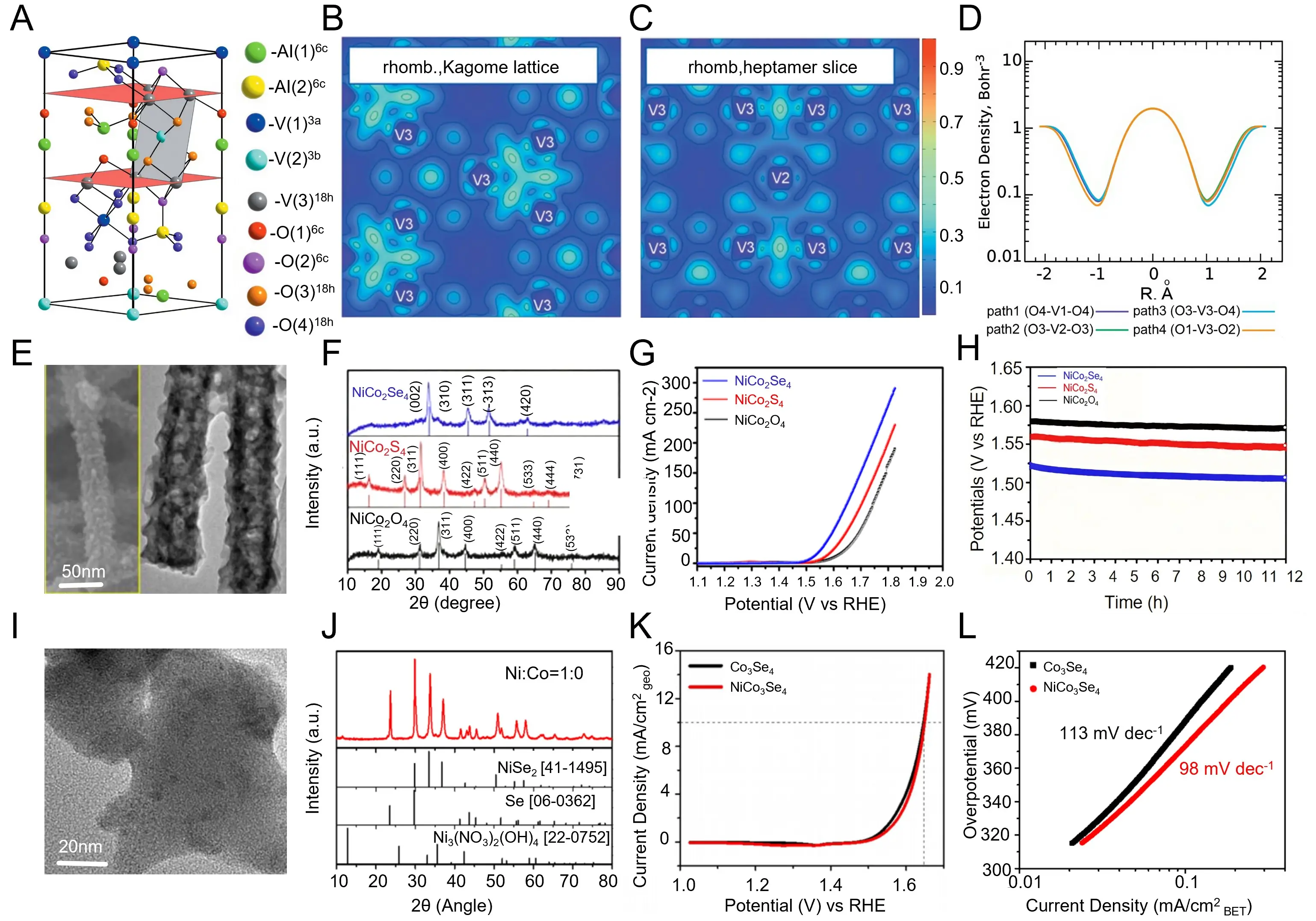
Figure 9. (a) Crystal structure of AlV2O4 showing the atomic positions of Al, V, and O in the spinel lattice; (b) ELF map showing the rhombohedral Kagome-like arrangement of V sites; (c) ELF slice showing heptamer-like V-site clustering under the rhombohedral distortion; (d) Line profiles of electron density along several V–O–V and V–V paths obtained from ELF analysis. Republished with permission from[221]; (e) SEM images of Ni, Co-based nanowires showing their one-dimensional morphology; (f) XRD patterns of NiCo2O4, NiCo2S4, and NiCo2Se4 showing the phase evolution after anion exchange; (g) Polarization curves comparing the OER performance of NiCo2O4, NiCo2S4, and NiCo2Se4 in 1 M KOH; (h) Polarization curves comparing OER activities of NiCo2O4, NiCo2S4 and NiCo2Se4 in 1 M KOH. Republished with permission from[213]; (i) HRTEM image of NiCo2Se4 showing nanoscale crystallinity; (j) XRD patterns of precursor Ni-Co selenide phases, including NiSe2 and associated intermediates; (k) OER polarization curves comparing NiCo2Se4 and Co3Se4; (l) Tafel plots and overpotential comparison of NiCo2Se4 and Co3Se4 measured in 1 M KOH. Republished with permission from[214]. ELF: electron localization function; OER: oxygen evolution reaction; SEM: scanning electron microscopy; XRD: X-ray diffraction; HRTEM: high-resolution transmission electron microscopy.
Building upon the previous discussion of anion-regulated selenide systems, Ganguli et al. synthesized monoclinic NiCo2Se4 and Co3Se4 nanoparticles via a one-pot solvothermal method using elemental selenium and NaBH4 under controlled reductive conditions[214]. As shown in Figure 9i, j, both catalysts exhibit uniform nanospherical morphologies with high crystallinity, where the PXRD profiles confirm the formation of the monoclinic C2/m phase with characteristic reflections of the (101) plane. The coexistence of Ni and Co in multiple valence states induces synergistic electronic coupling and optimizes the metal-selenium coordination environment. Electrochemical analyses reveal that NiCo2Se4 delivers a lower overpotential (≈ 400 mV @ 10 mA cm-2) and a smaller Tafel slope (98 mV dec-1) compared with Co3Se4 (113 mV dec-1), indicating accelerated oxygen-evolution kinetics and improved intrinsic activity (Figure 9k,l). This enhancement arises from the cooperative effect between Ni and Co centers, which promotes charge delocalization and optimizes adsorption energetics of oxygen intermediates. Overall, these findings demonstrate that monoclinic lattice distortion and bimetallic synergy effectively modulate the intrinsic electronic structure of selenides, providing a structural model for designing high-performance OER catalysts.
4. Summary, Challenges, and Perspectives
In this review, the crystal phase-dependent electrocatalytic behavior of spinel oxides is systematically summarized, with emphasis on how distinct crystal phases govern electrocatalytic performance. Through detailed analysis of phase-specific structural characteristics, the discussion elucidates the intrinsic relationships between crystal structure and catalytic properties, clarifying how variations in phase symmetry, electron transport pathways, surface reactivity, and structural stability collectively shape the catalytic activity of spinel oxides. The uniqueness of this review lies in its comprehensive summary of the basic characteristics of crystal phases, as well as its practical approach to regulating crystal phase distribution during spinel oxide synthesis to optimize electrocatalytic water splitting performance. Unlike traditional studies on electrocatalyst design, this review clarifies the advantages and disadvantages of different crystal phases, providing new insights for optimizing electrocatalysts and offering both theoretical and practical guidance for developing efficient and stable electrocatalytic water splitting materials. The phase-dependent structure–performance relationships can be summarized as follows: cubic spinels are structurally robust with continuous charge transport but typically require defect, strain, or interface regulation for activity gains, whereas hexagonal and tetragonal phases offer greater defect or distortion enabled tuning of adsorption energetics provided that stability is maintained, and other low-symmetry phases (orthorhombic, rhombohedral, monoclinic) can be highly active yet more prone to reconstruction or phase evolution and thus require stabilization. These phase trends underpin the challenges and perspectives discussed below.
Currently, spinel oxide electrocatalysts still face several challenges. First, although the impact of crystal phases on electrocatalytic performance has been widely discussed, it remains a significant challenge to precisely control the distribution of different crystal phases during synthesis. Existing synthesis methods often lead to difficulties in phase transformation and are highly sensitive to external conditions, making it challenging to obtain electrocatalysts with the desired structure and performance. Therefore, developing stable and controllable synthesis strategies that allow precise regulation of crystal phases under specific reaction conditions is key to enhancing electrocatalytic performance. Second, the long-term stability of electrocatalytic water splitting remains an unresolved issue. Although many spinel oxides exhibit good initial electrocatalytic activity, the catalyst’s crystal phase may change during prolonged reactions, leading to a decrease in activity. Under harsh operation (long-term electrolysis and high current densities), local pH gradients, bubble flux, and dissolution/redeposition can accelerate spinel surface reconstruction or near-surface phase transformation, so maintaining both phase integrity and fast kinetics at extreme rates remains challenging, even for catalysts stable for > 1,500 h at 200 mA cm-2. Future studies should combine long-duration/high-current testing with in situ/operando probes to track phase/valence evolution and identify the steady-state active surface beyond the pristine phase[197]. Thus, ensuring the stability of the material in complex reaction environments while enhancing activity is still a major challenge. Finally, environmental factors, such as temperature, pH, and solution composition, often play a crucial role but are frequently overlooked in experimental design. These factors are of paramount importance in practical applications, and it is essential to investigate their comprehensive impact on crystal phase transitions and electrocatalytic performance. Research into designing electrocatalytic materials that can adapt to various environmental conditions is an important direction for future work.
Looking ahead, with the continuous development of nanomaterial synthesis techniques and interface engineering, precise control over the crystal phases and surface properties of spinel oxides will become key to improving electrocatalytic performance. By combining precise phase regulation with surface functionalization, future research will enable the design of electrocatalysts with high efficiency, stability, and durability. Moreover, leveraging advanced computational techniques, such as first-principles calculations and machine learning, will allow researchers to gain deeper insights into the electronic behaviors and catalytic mechanisms of different crystal phase structures, providing more accurate theoretical guidance for the design of spinel oxide electrocatalysts. In parallel, emerging in situ/operando characterization (e.g., operando XAS/Raman and microscopy) can directly track phase/defect/valence evolution under working potentials, while high-throughput synthesis and screening, ideally coupled with data-driven models, can rapidly map composition–phase–activity/stability landscapes to accelerate rational phase engineering. Furthermore, future studies will focus on the development of multifunctional spinel oxides, allowing them to exhibit superior performance in a wider range of catalytic reactions, thus providing new technological breakthroughs for advancing green energy development and environmental protection.
Acknowledgements
ChatGPT-assisted tools were used for language editing only. The authors are responsible for the accuracy and scientific content of the article.
Authors contribution
Ma T, Yang M: Conceptualization, formal analysis, data curation, writing-original draft.
Feng J: Formal analysis, data curation, software, writing-original draft.
Zhou L: Software.
Xu W: Conceptualization, funding acquisition.
Mao E: Conceptualization, supervision, validation, writing-review & editing.
Wan J: Conceptualization, funding acquisition, supervision, validation, writing-review & editing.
Conflicts of interest
Jun Wan is a Youth Editorial Board Member of Smart Materials and Devices. The other authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (52203070), the Natural Science Foundation of Hubei Province (2025AFB863), and the Opening Project of Hubei Key Laboratory of Biomass Fibers and Eco-Dyeing & Finishing (STRZ202518).
Copyright
© The Author(s) 2026.
References
-
1. Ahmed MG, Tay YF, Chi X, Zhang M, Tan JMR, Chiam SY, et al. Efficient ternary Mn-based spinel oxide with multiple active sites for oxygen evolution reaction discovered via high-throughput screening methods. Small. 2023;19(2):e2204520.[DOI]
-
2. Bu F, Deng Y, Xu J, Yang D, Li Y, Li W, et al. Electrocatalytic reductive deuteration of arenes and heteroarenes. Nature. 2024;634(8034):592-599.[DOI]
-
3. Wang T, Cao X, Jiao L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality. 2022;1(1):21.[DOI]
-
4. Song X, Sun L, Gao P, Cui R, Han W, Huang X, et al. Unveiling the remarkable catalytic performance of Al2O3@Cu-Ce core–shell nanofiber catalyst for carbonyl sulfide hydrolysis at low temperature. EcoEnergy. 2025;3(3):e70011.[DOI]
-
5. Ahmed MG, Phang YF, Tay YF, Sadhu A, Mishra P, Yuwono AH, et al. Trimetallic spinel NiCo2−xMoxO4 oxygen evolution catalyst enabling bias-free solar water splitting with inverted perovskite solar cells. J Mater Chem A. 2025;13(19):14172-14180.[DOI]
-
6. Breuer O, Chakraborty A, Liu J, Kravchuk T, Burstein L, Grinblat J, et al. Understanding the role of minor molybdenum doping in LiNi0.5Co0.2Mn0.3O2 electrodes: From structural and surface analyses and theoretical modeling to practical electrochemical cells. ACS Appl Mater Interfaces. 2018;10(35):29608-29621.[DOI]
-
7. Sun L, Feng M, Peng Y, Zhao X, Shao Y, Yue X, et al. Constructing oxygen vacancies by doping Mo into spinel Co3O4 to trigger a fast oxide path mechanism for acidic oxygen evolution reaction. J Mater Chem A. 2024;12(15):8796-8804.[DOI]
-
8. Cao K, Yuan G, Liu M, Lu S, Shin BC, Yu L. Organoselenium catalyzed reaction: Sustainable chemistry from laboratory to industry. Sustain Eng Novit. 2025;1(1):1.[DOI]
-
9. Wang YH, Zheng S, Yang WM, Zhou RY, He QF, Radjenovic P, et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature. 2021;600(7887):81-85.[DOI]
-
10. Chandrasekaran S, Bowen C, Zhang P, Li Z, Yuan Q, Ren X, et al. Spinel photocatalysts for environmental remediation, hydrogen generation, CO2 reduction and photoelectrochemical water splitting. J Mater Chem A. 2018;6(24):11078-11104.[DOI]
-
11. Sun S, Sun Y, Zhou Y, Shen J, Mandler D, Neumann R, et al. Switch of the rate-determining step of water oxidation by spin-selected electron transfer in spinel oxides. Chem Mater. 2019;31(19):8106-8111.[DOI]
-
12. Cheng X, Li T, Yan L, Jiao Y, Zhang Y, Wang K, et al. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil-water separation. Sci Adv. 2023;9(34):eadh8195.[DOI]
-
13. Xie H, Shao Z. A practical method for splitting seawater into hydrogen fuel. Nature. 2022.[DOI]
-
14. Sun Y, Wang J, Xi S, Shen J, Luo S, Ge J, et al. Navigating surface reconstruction of spinel oxides for electrochemical water oxidation. Nat Commun. 2023;14(1):2467.[DOI]
-
15. Zhang Q, Song Z, Sun X, Liu Y, Wan J, Betzler SB, et al. Atomic dynamics of electrified solid-liquid interfaces in liquid-cell TEM. Nature. 2024;630(8017):643-647.[DOI]
-
16. Chu L, Shen H, Wei H, Chen H, Ma G, Yan W. Morphology engineering of ZnO micro/nanostructures under mild conditions for optoelectronic application. Int J Miner Metall Mater. 2025;32(2):498-503.[DOI]
-
17. Tian H, Zhang H, Zhu Z, Dai J, Qin S, Xu R, et al. Near-unity solar reflectance and mid-infrared transparency via microwave-engineered 2D Y2O3 for passive radiative cooling. J Mater Chem A. 2025;13(45):39330-39339.[DOI]
-
18. Dai J, Wang M, Tian H, Fan W, Liu K, Xu W, et al. Microwave shock-driven thermal engineering of unconventional cubic 2D LaMnO3 for efficient oxygen evolution. J Mater Chem A. 2025;13(37):31002-31012.[DOI]
-
19. Du F, Yang T, Hao H, Li S, Xu C, Yao T, et al. Giant electrocaloric effect in high-polar-entropy perovskite oxides. Nature. 2025;640(8060):924-930.[DOI]
-
20. Sun YY, Zhang XY, Tang J, Li X, Fu HQ, Xu HG, et al. Amorphous oxysulfide reconstructed from spinel NiCo2 S4 for efficient water oxidation. Small. 2023;19(27):e2207965.[DOI]
-
21. Du X, Huang J, Zhang J, Yan Y, Wu C, Hu Y, et al. Modulating electronic structures of inorganic nanomaterials for efficient electrocatalytic water splitting. Angew Chem Int Ed. 2019;58(14):4484-4502.[DOI]
-
22. Dai JA, Xian J, Liu K, Wu Z, Fan M, Qin S, et al. Unconventional metastable cubic 2D LaMnO3 for efficient alkaline seawater oxygen evolution. Chin J Catal. 2025;74:228-239.[DOI]
-
23. Takata T, Jiang J, Sakata Y, Nakabayashi M, Shibata N, Nandal V, et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature. 2020;581(7809):411-414.[DOI]
-
24. Dey GR, Teklu S, Shi Z, Hu M, Thompson KL, Soliman SS, et al. Structural, compositional, and morphological interrelationships in rocksalt (FeCoMnMgZn)O high entropy oxide nanocrystals for oxygen evolution electrocatalysis. J Am Chem Soc. 2025;147(28):24230-24234.[DOI]
-
25. Einert M, Waheed A, Lauterbach S, Mellin M, Rohnke M, Wagner LQ, et al. Sol-gel-derived ordered mesoporous high entropy spinel ferrites and assessment of their photoelectrochemical and electrocatalytic water splitting performance. Small. 2023;19(14):e2205412.[DOI]
-
26. Dey S, Masero F, Brack E, Fontecave M, Mougel V. Electrocatalytic metal hydride generation using CPET mediators. Nature. 2022;607(7919):499-506.[DOI]
-
27. Zhu Y, Liu X, Jin S, Chen H, Lee W, Liu M, et al. Anionic defect engineering of transition metal oxides for oxygen reduction and evolution reactions. J Mater Chem A. 2019;7(11):5875-5897.[DOI]
-
28. Tao L, Guo P, Zhu W, Li T, Zhou X, Fu Y, et al. Highly efficient mixed-metal spinel cobaltite electrocatalysts for the oxygen evolution reaction. Chin J Catal. 2020;41(12):1855-1863.[DOI]
-
29. Wang X, Xi S, Huang P, Du Y, Zhong H, Wang Q, et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature. 2022;611(7937):702-708.[DOI]
-
30. Ding M, Ji D, Hu W. Degradable organic neuromorphic transistors: Emerging progress in optoelectronically co-reconfigurable devices for sustainable electronics. FlexMat. 2025;2(4):510-532.[DOI]
-
31. Tao L, Wang Y, Zou Y, Zhang N, Zhang Y, Wu Y, et al. Charge transfer modulated activity of carbon-based electrocatalysts. Adv Energy Mater. 2020;10(11):1901227.[DOI]
-
32. Ding S, Duan J, Chen S. Recent advances of metal suboxide catalysts for carbon-neutral energy applications. EcoEnergy. 2024;2(1):45-82.[DOI]
-
33. Teitsworth TS, Hill DJ, Litvin SR, Ritchie ET, Park JS, Custer JP, et al. Water splitting with silicon p–i–n superlattices suspended in solution. Nature. 2023;614(7947):270-274.[DOI]
-
34. Badalyan A, Stahl SS. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature. 2016;535(7612):406-410.[DOI]
-
35. Xie H, Zhao Z, Liu T, Wu Y, Lan C, Jiang W, et al. A membrane-based seawater electrolyser for hydrogen generation. Nature. 2022;612(7941):673-678.[DOI]
-
36. Hu R, Wei L, Xian J, Fang G, Wu Z, Fan M, et al. Microwave shock process for rapid synthesis of 2D porous La0.2Sr0.8CoO3 perovskite as an efficient oxygen evolution reaction catalyst. Acta Phys Chim Sin. 2023;39(9):2212025.[DOI]
-
37. Zheng Y, Hussain G, Zheng C, Zhou X, Zhang M, Xie S, et al. Impact of the rhenium substitution on the oxygen evolution reaction of spinel CoFe2O4. J Mater Chem A. 2024;12(30):19521-19531.[DOI]
-
38. Wang J, Xu K, Yu Z, Cui H, Huang H, Zhang C, et al. Lead-doped ruthenium-iridium oxide catalysts for durable acidic oxygen evolution in proton exchange membrane electrolyzers at 3 A cm-2. Mater Futur. 2026;5(1):015101.[DOI]
-
39. Zhu P, Wu ZY, Elgazzar A, Dong C, Wi TU, Chen FY, et al. Continuous carbon capture in an electrochemical solid-electrolyte reactor. Nature. 2023;618(7967):959-966.[DOI]
-
40. Zheng Y, Ma M, Shao H. Recent advances in efficient and scalable solar hydrogen production through water splitting. Carbon Neutrality. 2023;2(1):23.[DOI]
-
41. Zhong H, Zeng C, Lai J, Liu G, Jin L, Liu C, et al. Enhanced oxygen evolution by activating vacancy defects on metal–organic framework-derived Co3O4/NC. Carbon Neutralization. 2025;4(4):e70030.[DOI]
-
42. Huang J, Tian H, Zhang H, Huang Z, Long Y, Xu W, et al. Engineering materials and structural paradigms for mid-infrared thermal management. Mater Today Energy. 2025;52:101944.[DOI]
-
43. Bai J, Cheng W, Wang T, Liu S. Synergistic regulation of lithium nucleation and anion-rich solvation structure via silver trifluoroacetate additive for stable lithium metal anodes. Smart Mater Devices. 2025;1:202534.[DOI]
-
44. Chen H, Liang X, Liu Y, Ai X, Asefa T, Zou X. Active site engineering in porous electrocatalysts. Adv Mater. 2020;32(44):e2002435.[DOI]
-
45. Tian Y, Zhang J, Bin Z. Triazolotriazine-based thermally activated delayed fluorescence sensitizer for narrowband red fluorescence OLEDs. Smart Mater Devices. 2025;1:38642.[DOI]
-
46. Huang J, Tian H, Zhang H, Zhu Z, Qin S, Xu W, et al. Two-dimensional materials for enhanced mid-infrared thermal management. 2D Mater. 2025;12(3):032003.[DOI]
-
47. Upale P, Verma S, Ogale SB. Superior oxygen evolution reaction performance of NiCoFe spinel oxide nanowires in situ grown on β-Ni(OH)2 nanosheet-decorated Ni foam: Case studies on stoichiometric and off-stoichiometric oxides. J Mater Chem A. 2023;11(16):8972-8987.[DOI]
-
48. Huang Y, Li M, Pan F, Zhu Z, Sun H, Tang Y, et al. Plasma-induced Mo-doped Co3O4 with enriched oxygen vacancies for electrocatalytic oxygen evolution in water splitting. Carbon Energy. 2023;5(3):e279.[DOI]
-
49. Hussain M, Abu El Maaty L, Alomar MA, Ali M, Abdullah M, Aman S, et al. Improvement of spinel OER electrochemical property by doping strategy for water splitting. Appl Phys A. 2024;130(8):554.[DOI]
-
50. Indra A, Menezes PW, Das C, Göbel C, Tallarida M, Schmeiβer D, et al. A facile corrosion approach to the synthesis of highly active CoOx water oxidation catalysts. J Mater Chem A. 2017;5(10):5171-5177.[DOI]
-
51. Ji B, Wang Y, Liu Y, Zhao Y, Xu F, Huang J, et al. Scalable and healable gradient textiles for multi-scenario radiative cooling via bicomponent blow spinning. Nanomicro Lett. 2025;18(1):86.[DOI]
-
52. Zhang C, Gao S, Chen X, Yu D, Wang L, Fan X, et al. Facile preparation of alkali metal-modified hollow nanotubular manganese-based oxide catalysts and their excellent catalytic soot combustion performance. Smart Mol. 2025;3(1):e20240022.[DOI]
-
53. Ji J, Hou Y, Zhou S, Qiu T, Zhang L, Ma L, et al. Oxygen-coordinated low-nucleus cluster catalysts for enhanced electrocatalytic water oxidation. Carbon Energy. 2023;5(2):e216.[DOI]
-
54. Jiang H, Li J, Xiao Z, Wang B, Fan M, Xu S, et al. The rapid production of multiple transition metal carbides via microwave combustion under ambient conditions. Nanoscale. 2020;12(30):16245-16252.[DOI]
-
55. Zhang J, Fu X, Kwon S, Chen K, Liu X, Yang J, et al. Tantalum-stabilized ruthenium oxide electrocatalysts for industrial water electrolysis. Science. 2025;387(6729):48-55.[DOI]
-
56. Jiang H, Xian J, Hu R, Mi S, Wei L, Fang G, et al. Microwave discharge for rapid introduction of bimetallic-synergistic configuration to conductive catecholate toward long-term supercapacitor. Chem Eng J. 2023;455:140804.[DOI]
-
57. Yao Q, Liu P, Yang F, Zhu Y, Pan Y, Xue H, et al. Ferroelectric polarization in Bi0.9Dy0.1FeO3/g-C3N4 Z-scheme heterojunction boosts photocatalytic hydrogen evolution. Sci China Mater. 2024;67(10):3160-3167.[DOI]
-
58. Kashyap V, Pandikassala A, Singla G, Khan TS, Ali Haider M, Vinod CP, et al. Unravelling faradaic electrochemical efficiencies over Fe/Co spinel metal oxides using surface spectroscopy and microscopy techniques. Nanoscale. 2022;14(42):15928-15941.[DOI]
-
59. Zhou P, Navid IA, Ma Y, Xiao Y, Wang P, Ye Z, et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature. 2023;613(7942):66-70.[DOI]
-
60. Katzbaer RR, Dos Santos Vieira FM, Dabo I, Mao Z, Schaak RE. Band gap narrowing in a high-entropy spinel oxide semiconductor for enhanced oxygen evolution catalysis. J Am Chem Soc. 2023;145(12):6753-6761.[DOI]
-
61. He B, Bai F, Jain P, Li T. A review of surface reconstruction and transformation of 3d transition-metal (oxy)Hydroxides and spinel-type oxides during the oxygen evolution reaction. Small. 2025;21(10):e2411479.[DOI]
-
62. Khalid R, Tahir M, Umar M, Fang P, Li Y. The newly discovered pathway for oxygen evolution reaction: In-situ/operando characterization techniques for catalyst development. Carbon Energy. 2025;10(7):e70067.[DOI]
-
63. Xu R, Zhu Z, Zhang H, Fan W, Guan T, Wei Y, et al. Synergistic material-structure engineering for mid-infrared thermal management in textiles. Small. 2025;21(47):e09257.[DOI]
-
64. He Q, Sheng B, Zhu K, Zhou Y, Qiao S, Wang Z, et al. Phase engineering and synchrotron-based study on two-dimensional energy nanomaterials. Chem Rev. 2023;123(17):10750-10807.
-
65. Vedanarayanan M, Pitchai C, Chen CM. Exploring spinel oxides from bimetallic to high-entropy with a focus on the structure and performance in the oxygen evolution reaction. J Mater Chem A. 2025;13(24):18040-18061.[DOI]
-
66. He Y, Zhang J, He G, Han X, Zheng X, Zhong C, et al. Ultrathin Co3O4 nanofilm as an efficient bifunctional catalyst for oxygen evolution and reduction reaction in rechargeable zinc-air batteries. Nanoscale. 2017;9(25):8623-8630.[DOI]
-
67. Hong JS, Seo H, Lee YH, Cho KH, Ko C, Park S, et al. Nickel-doping effect on Mn3O4 nanoparticles for electrochemical water oxidation under neutral condition. Small Meth. 2020;4(3):1900733.[DOI]
-
68. Zhao ZJ, Liu S, Zha S, Cheng D, Studt F, Henkelman G, et al. Theory-guided design of catalytic materials using scaling relationships and reactivity descriptors. Nat Rev Mater. 2019;4(12):792-804.[DOI]
-
69. Hoque MA, Gerken JB, Stahl SS. Synthetic dioxygenase reactivity by pairing electrochemical oxygen reduction and water oxidation. Science. 2024;383(6679):173-178.[DOI]
-
70. Yoshinaga T, Saruyama M, Xiong A, Ham Y, Kuang Y, Niishiro R, et al. Boosting photocatalytic overall water splitting by Co doping into Mn3O4 nanoparticles as oxygen evolution cocatalysts. Nanoscale. 2018;10(22):10420-10427.[DOI]
-
71. Kim D, Thaheem I, Yu H, Park JH, Lee KT. Highly promoted electrocatalytic activity of spinel CoFe2O4 by combining with Er0.4Bi1.6O3 as a bifunctional oxygen electrode for reversible solid oxide cells. J Mater Chem A. 2022;10(4):2045-2054.[DOI]
-
72. Kim JH, Jang YJ, Kim JH, Jang JW, Choi SH, Lee JS. Defective ZnFe₂O₄ nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale. 2015;7(45):19144-19151.[DOI]
-
73. Yu A, Kim MH, Lee C, Lee Y. Structural transformation between rutile and spinel crystal lattices in Ru-Co binary oxide nanotubes: Enhanced electron transfer kinetics for the oxygen evolution reaction. Nanoscale. 2021;13(32):13776-13785.[DOI]
-
74. Kim JH, Kim HE, Kim JH, Lee JS. Ferrites: Emerging light absorbers for solar water splitting. J Mater Chem A. 2020;8(19):9447-9482.[DOI]
-
75. Zhang N, Cheng F, Liu Y, Zhao Q, Lei K, Chen C, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J Am Chem Soc. 2016;138(39):12894-12901.[DOI]
-
76. Kim SY, Yu A, Lee Y, Kim HY, Kim YJ, Lee NS, et al. Single phase of spinel Co2RhO4 nanotubes with remarkably enhanced catalytic performance for the oxygen evolution reaction. Nanoscale. 2019;11(19):9287-9295.[DOI]
-
77. Kwon S, Lee HE, Han D, Lee JH. Low-temperature fabrication of crystalline MnCoO spinel film on porous carbon paper for efficient oxygen evolution reaction. Chem Commun. 2021;57(29):3595-3598.[DOI]
-
78. Lee SA, Kim J, Kwon KC, Park SH, Jang HW. Anion exchange membrane water electrolysis for sustainable large-scale hydrogen production. Carbon Neutralization. 2022;1(1):26-48.[DOI]
-
79. Lei J, Wang Z, Zhang Y, Ju M, Fei H, Wang S, et al. Understanding and resolving the heterogeneous degradation of anion exchange membrane water electrolysis for large-scale hydrogen production. Carbon Neutrality. 2024;3(1):25.[DOI]
-
80. Zhao H, Yuan ZY. Engineering of transition metal phosphide-based heterostructures for electrocatalytic water splitting. Smart Mater Devices. 2025;1:202521.[DOI]
-
81. Chong L, Gao G, Wen J, Li H, Xu H, Green Z, et al. La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science. 2023;380(6645):609-616.[DOI]
-
82. Wang H, Li X, Deng Y, Jiang J, Ma H, Zou J. Advances in MXene-based single-atom catalysts for electrocatalytic applications. Coord Chem Rev. 2025;529:216462.[DOI]
-
83. Li A, Kong S, Adachi K, Ooka H, Fushimi K, Jiang Q, et al. Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis. Science. 2024;384(6696):666-670.[DOI]
-
84. Xiao X, Huang D, Fu Y, Wen M, Jiang X, Lv X, et al. Engineering NiS/Ni2P heterostructures for efficient electrocatalytic water splitting. ACS Appl Mater Interfaces. 2018;10(5):4689-4696.[DOI]
-
85. Li X, Wei X, Wang X, Lou C, Zhang W, Xu J, et al. B-site mixed cationic tetrahedral layer confined the concentration and mobility of interstitial oxygen in mellite family. J Mater Chem A. 2023;11(11):5615-5626.[DOI]
-
86. Feng Y, Wang M, Zhang H, Qin S, Guan T, Chang B, et al. Engineering electrocatalytic structures through molten salt-mediated mechanistic control. EcoEnergy. 2025;3(4):e70022.[DOI]
-
87. Zander J, Marschall R. 1 Min synthesis of phase pure nanocrystalline high-entropy sulfides for efficient water electrolysis. EcoEnergy. 2025;3(2):482-498.[DOI]
-
88. Li Y, Zhang X, Li T, Chen Y, Liu Y, Feng L. Accelerating materials discovery for electrocatalytic water oxidation via center-environment deep learning in spinel oxides. J Mater Chem A. 2024;12(30):19362-19377.[DOI]
-
89. Friebel D, Louie MW, Bajdich M, Sanwald KE, Cai Y, Wise AM, et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J Am Chem Soc. 2015;137(3):1305-1313.[DOI]
-
90. Wang Y, Liu Y, Guo Y. Stretchable organic transistors for bioinspired electronics: Materials, devices and applications. FlexMat. 2025;2(3):312-340.[DOI]
-
91. Li Z, Wang Y, Jin Z. Metal-free perovskites for X-ray detection and imaging: Progress and prospects. EcoEnergy. 2025;3(4):e70013.[DOI]
-
92. Han Y, Wang J, Liu Y, Li T, Wang T, Li X, et al. Stability challenges and opportunities of NiFe-based electrocatalysts for oxygen evolution reaction in alkaline media. Carbon Neutralization. 2024;3(2):172-198.[DOI]
-
93. Shi W, Shen T, Xing C, Sun K, Yan Q, Niu W, et al. Ultrastable supported oxygen evolution electrocatalyst formed by ripening-induced embedding. Science. 2025;387(6735):791-796.[DOI]
-
94. Hao W, Huang G, Ma X, Lei F, Zhang Q, Zhang J, et al. Economical iron-based catalyst electrode for highly stable catalytic industrial-scale overall seawater splitting. Carbon Neutrality. 2024;3(1):37.[DOI]
-
95. Liu F, Liu J, Gong J, Lin R, Qiu S, Liu Z, et al. Complex-mediated nucleophilic aromatic substitution with aryl nitriles to realize intramolecular flapping-restricted D-a AIEgens for bioimaging. Smart Mol. 2025;3(2):e20240039.[DOI]
-
96. Liu J, Yang X, Si F, Zhao B, Xi X, Wang L, et al. Interfacial component coupling effects towards precise heterostructure design for efficient electrocatalytic water splitting. Nano Energy. 2022;103:107753.[DOI]
-
97. Liu J, Yuan H, Wang Z, Li J, Yang M, Cao L, et al. Self-supported nickel iron oxide nanospindles with high hydrophilicity for efficient oxygen evolution. Chem Commun. 2019;55(73):10860-10863.[DOI]
-
98. Zhang Y, Wu J, Guo B, Huo H, Niu S, Li S, et al. Recent advances of transition-metal metaphosphates for efficient electrocatalytic water splitting. Carbon Energy. 2023;5(12):e375.[DOI]
-
99. Liu M, Pang Y, Zhang B, de Luna P, Voznyy O, Xu J, et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature. 2016;537(7620):382-386.[DOI]
-
100. Guo P, Liu D, Wang Q, Chen P, Yang H, Zhang M, et al. Balancing the surface reconstruction of spinel oxides for efficient oxygen evolution reaction. Nano Lett. 2025;25(25):10209-10217.[DOI]
-
101. Liu R, Wei Z, Peng L, Zhang L, Zohar A, Schoeppner R, et al. Establishing reaction networks in the 16-electron sulfur reduction reaction. Nature. 2024;626(7997):98-104.[DOI]
-
102. Guo S, Wu H, Puleo F, Liotta L. B-site metal (Pd, Pt, Ag, Cu, Zn, Ni) promoted La1–xSrxCo1–yFeyO3–δ perovskite oxides as cathodes for IT-SOFCs. Catalysts. 2015;5(1):366-391.[DOI]
-
103. Guo Y, Zhang N, Wang X, Qian Q, Zhang S, Li Z, et al. A facile spray pyrolysis method to prepare Ti-doped ZnFe2O4 for boosting photoelectrochemical water splitting. J Mater Chem A. 2017;5(16):7571-7577.[DOI]
-
104. Zhang L, Jia J, Yan J. Challenges and strategies for synthesizing high performance micro and nanoscale high entropy oxide materials. Small. 2024;20(28):e2309586.[DOI]
-
105. Shi L, Zhang W, Li J, Yan Q, Chen Z, Zhou X, et al. Recent development of non-iridium-based electrocatalysts for acidic oxygen evolution reaction. Carbon Neutralization. 2024;3(6):1101-1130.[DOI]
-
106. Zha J, Huang H, Zhang Q, Tan C. Materials innovation for circularly polarized photodetectors. Smart Mater Devices. 2025;1:202501.[DOI]
-
107. Han N, Zhang W, Guo W, Pan H, Jiang B, Xing L, et al. Designing oxide catalysts for oxygen electrocatalysis: Insights from mechanism to application. Nanomicro Lett. 2023;15(1):185.[DOI]
-
108. Liu X, Chang Z, Luo L, Xu T, Lei X, Liu J, et al. Hierarchical ZnxCo3–xO4 nanoarrays with high activity for electrocatalytic oxygen evolution. Chem Mater. 2014;26(5):1889-1895.[DOI]
-
109. Zhang J, Liu C, Zhang B. Insights into single-atom metal–support interactions in electrocatalytic water splitting. Small Meth. 2019;3(9):1800481.[DOI]
-
110. Cai J, Wu Z, Wang S, Guo J, Fan M, Xu W, et al. Exploring advanced microwave strategy for the synthesis of two-dimensional energy materials. Appl Phys Rev. 2024;11(4):041320.[DOI]
-
111. You B, Tang MT, Tsai C, Abild-Pedersen F, Zheng X, Li H. Enhancing electrocatalytic water splitting by strain engineering. Adv Mater. 2019;31(17):e1807001.[DOI]
-
112. Guo C, Shi Y, Lu S, Yu Y, Zhang B. Amorphous nanomaterials in electrocatalytic water splitting. Chin J Catal. 2021;42(8):1287-1296.[DOI]
-
113. Liu Y, Wang W, Xu X, Marcel Veder JP, Shao Z. Recent advances in anion-doped metal oxides for catalytic applications. J Mater Chem A. 2019;7(13):7280-7300.[DOI]
-
114. Wu Z, Wang X, Huang J, Gao F. A Co-doped Ni–Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting. J Mater Chem A. 2018;6(1):167-178.[DOI]
-
115. Cady CW, Gardner G, Maron ZO, Retuerto M, Go YB, Segan S, et al. Tuning the electrocatalytic water oxidation properties of AB2O4 spinel nanocrystals: A (Li, Mg, Zn) and B (Mn, Co) site variants of LiMn2O4. ACS Catal. 2015;5(6):3403-3410.[DOI]
-
116. Rong C, Sun Q, Zhu J, Arandiyan H, Shao Z, Wang Y, et al. Advances in stabilizing spinel cobalt oxide-based catalysts for acidic oxygen evolution reaction. Adv Sci. 2025;12(35):e09415.[DOI]
-
117. Lu Y, Wang H, Li Q, Liu Q, Zhang X, Jia Y, et al. Ru@NiMoS aggregate with boosted electrochemical catalysis for enhanced electrochemiluminescence and lidocaine detection. Smart Mol. 2025;3(1):e20240011.[DOI]
-
118. Yang T, Yan Y, Liu R, Huang K, Xu R, Chen J, et al. Engineering twins within lattice-matched Co/CoO heterostructure enables efficient hydrogen evolution reactions. Nano Lett. 2025;25(19):7707-7715.[DOI]
-
119. Guo M, Zhang Z, Zhang H, Lin J, Qin X, Deng R, et al. Dual-phase spinel manganese-cobalt hybrid oxide for enhanced oxygen evolution catalysis in acid media. J Energy Chem. 2025;108:361-372.[DOI]
-
120. Zhang X, Yan Y, Liu Y, Li C, Cao J, Qi J. CO2-affinitive surface of metal/Nafion attributable to selective polarization for superior CO2RR. Green Chem. 2025;27(47):15096-15105.[DOI]
-
121. Lv XW, Tian WW, Yuan ZY. Recent advances in high-efficiency electrocatalytic water splitting systems. Electrochem Energy Rev.2023;6(1):23.[DOI]
-
122. Lyu Y, Zheng J, Xiao Z, Zhao S, Jiang SP, Wang S. Identifying the intrinsic relationship between the restructured oxide layer and oxygen evolution reaction performance on the cobalt pnictide catalyst. Small. 2020;16(14):e1906867.[DOI]
-
123. Ma J, Luo W, Wang X, Yu X, Wang JJ, Hu H, et al. Saturated alcohols electrocatalytic oxidations on Ni-co bimetal oxide featuring balanced B- and L-acidic active sites. Nanomicro Lett. 2025;18(1):39.[DOI]
-
124. Ma X, Wu Z, Tian H, Fang G, Dai J, Ding T, et al. Durable coaxial fiber-based underwater strain sensor with reversible dry–wet transition. InfoMat. 2025;7(8):e70030.[DOI]
-
125. Maiyalagan T, Chemelewski KR, Manthiram A. Role of the morphology and surface planes on the catalytic activity of spinel LiMn1.5Ni0.5O4 for oxygen evolution reaction. ACS Catal. 2014;4(2):421-425.[DOI]
-
126. Feng Y, Dai J, Wang M, Ding W, Zhang H, Xu W, et al. Unraveling metastable perovskite oxides insights from structural engineering to synthesis paradigms. Microstructures. 2025;5(4):2025068.[DOI]
-
127. Manivasakan P, Ramasamy P, Kim J. Use of urchin-like Ni(x)Co(3-x)O4 hierarchical nanostructures based on non-precious metals as bifunctional electrocatalysts for anion-exchange membrane alkaline alcohol fuel cells. Nanoscale. 2014;6(16):9665-9672.[DOI]
-
128. Gou J, Lei X, Ji B, Zhang S, Zheng Y, Tang Y. Adaptive morphing of hydroxyl groups on covalency competing spinel oxides boosting oxygen evolution reactions. ACS Catal. 2025;15(3):2053-2062.[DOI]
-
129. Zhao Q, Yan Z, Chen C, Chen J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem Rev. 2017;117(15):10121-10211.[DOI]
-
130. Mefford JT, Akbashev AR, Kang M, Bentley CL, Gent WE, Deng HD, et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature. 2021;593(7857):67-73.[DOI]
-
131. Yan Y, Zhao C, Hui J, Zhang X, Zhang X, Chen P, et al. Molecular nonchemically amplified resists based on spirobixanthene backbone: Sulfoxime oxime esters versus sulfonium salts. Smart Mol. 2025;3(4):e70028.[DOI]
-
132. Gong Y, Yao J, Wang P, Li Z, Zhou H, Xu C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin J Chem Eng. 2022;43:282-296.[DOI]
-
133. Miao R, He J, Sahoo S, Luo Z, Zhong W, Chen SY, et al. Reduced graphene oxide supported nickel–manganese–cobalt spinel ternary oxide nanocomposites and their chemically converted sulfide nanocomposites as efficient electrocatalysts for alkaline water splitting. ACS Catal. 2017;7(1):819-832.[DOI]
-
134. Sun M, Tang Y, Wang J. Water oxidation in medium-entropy spinel oxides via lattice oxygen activation. ACS Energy Lett. 2025;10(11):5422-5430.[DOI]
-
135. Mo Y, Guan X, Wang S, Duan X. Oriented catalysis through chaos: High-entropy spinels in heterogeneous reactions. Chem Sci. 2025;16(4):1652-1676.[DOI]
-
136. Feng L, Yang S, Zha C, Yin Y, Wang L. Light-emitting diodes enabled by two-dimensional semiconductors: Architectures, optimization, and functional advances. FlexMat. 2025;2(3):420-441.[DOI]
-
137. Zhang L, Yang C, Xie Z, Wang X. Cobalt manganese spinel as an effective cocatalyst for photocatalytic water oxidation. Appl Catal B Environ. 2018;224:886-894.[DOI]
-
138. Moltved KA, Kepp KP. The chemical bond between transition metals and oxygen: Electronegativity, d-orbital effects, and oxophilicity as descriptors of metal–oxygen interactions. J Phys Chem C. 2019;123(30):18432-18444.[DOI]
-
139. Cai M, Cai G, Liu K, Wang D, Zhao H, He P. Ultralow Ru loading on spinel oxide enhances oxygen coupling for efficient acidic water oxidation. J Am Chem Soc. 2025;147(43):39953-39963.[DOI]
-
140. Mushtaq MA, Ahmad M, Shaheen A, Mehmood A, Yasin G, Arif M, et al. Advancing the development of hollow micro/nanostructured materials for electrocatalytic water splitting: Current state, challenges, and perspectives. ACS Mater Lett. 2024;6(7):3090-3111.[DOI]
-
141. Natarajan K, Munirathinam E, Yang TCK. operando investigation of structural and chemical origin of Co3O4 stability in acid under oxygen evolution reaction. ACS Appl Mater Interfaces. 2021;13(23):27140-27148.[DOI]
-
142. Lu YC, Liang W, Karasev A, Wang G, Wang C. Toward fossil-free steelmaking: Overview of physiochemical properties of hydrochar and its application in blast furnace and electric arc furnace processes. cScience. 2025;1:e70004.[DOI]
-
143. Nellist MR, Laskowski FA, Lin F, Mills TJ, Boettcher SW. Semiconductor-electrocatalyst interfaces: Theory, experiment, and applications in photoelectrochemical water splitting. Acc Chem Res. 2016;49(4):733-740.[DOI]
-
144. Yang H, Hu F, Zhang Y, Shi L, Wang Q. Controlled synthesis of porous spinel cobalt manganese oxides as efficient oxygen reduction reaction electrocatalysts. Nano Res. 2016;9(1):207-213.[DOI]
-
145. Nong HN, Falling LJ, Bergmann A, Klingenhof M, Tran HP, Spöri C, et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature. 2020;587(7834):408-413.[DOI]
-
146. Wen T, Zheng Y, Wang H, Zou J. Constructing black phosphorus nanosheet/CoNiSe2 nanoflower heterojunction with effective built-in electric field for robust H2 evolution in anion exchange membrane water electrolyzer. Appl Catal B Environ Energy. 2025;378:125588.[DOI]
-
147. Fang G, Ma X, Hu R, Dai J, Tian H, Li L, et al. Horizontally oriented 2D skin structures on fiber interface for long-life flexible energy storage devices. Chem Eng J. 2025;509:161557.[DOI]
-
148. Fu R, Wang L, Yang X, Li C, Ouyang M, Wu H, et al. Defects-engineered metal-organic frameworks for supercapacitor platform. Sustain Eng Novit. 2025;1(1):2.[DOI]
-
149. Yin J, Zhou P, An L, Huang L, Shao C, Wang J, et al. Self-supported nanoporous NiCo2O4 nanowires with cobalt-nickel layered oxide nanosheets for overall water splitting. Nanoscale. 2016;8(3):1390-1400.[DOI]
-
150. Nsanzimana JMV, Cai L, Jiang Z, Xia BY, Maiyalagan T. Engineering bifunctional catalytic microenvironments for durable and high-energy-density metal-air batteries. Nanomicro Lett. 2025;17(1):294.[DOI]
-
151. Olowoyo JO, Kriek RJ. Recent progress on bimetallic-based spinels as electrocatalysts for the oxygen evolution reaction. Small. 2022;18(41):e2203125.[DOI]
-
152. Wu Z, Fan M, Jiang H, Dai J, Liu K, Hu R, et al. Harnessing the unconventional cubic phase in 2D LaNiO3 perovskite for highly efficient urea oxidation. Angew Chem Int Ed. 2025;64(1):e202413932.[DOI]
-
153. Oener SZ, Foster MJ, Boettcher SW. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science. 2020;369(6507):1099-1103.[DOI]
-
154. Xiao Z, Xiao X, Kong LB, Dong H, Li X, He B, et al. Preparation of MXene-based hybrids and their application in neuromorphic devices. Int J Extreme Manuf. 2024;6(2):022006.[DOI]
-
155. Fu X, Shi R, Jiao S, Li M, Li Q. Structural design for electrocatalytic water splitting to realize industrial-scale deployment: Strategies, advances, and perspectives. J Energy Chem. 2022;70:129-153.[DOI]
-
156. Zhang Y, Wu J, Li T, Li H, Zhang Y, Wu X, et al. Efficiently enhanced selectivity of electrocatalyzing ethanol to high value-added acetaldehyde through tuning the cobalt valence state. ACS Catal. 2024;14(3):1706-1713.[DOI]
-
157. Oh S, Jung S, Lee YH, Song JT, Kim TH, Nandi DK, et al. Hole-selective CoOx/SiOx/Si heterojunctions for photoelectrochemical water splitting. ACS Catal. 2018;8(10):9755-9764.[DOI]
-
158. Wang M, Feng X, Li S, Ma Y, Peng Y, Yang S, et al. Spinel-type metal oxides with tailored amorphous/crystalline heterointerfaces for enhanced electrocatalytic water splitting. Adv Funct Mater. 2024;34(51):2410439.[DOI]
-
159. Wei C, Feng Z, Scherer GG, Barber J, Shao-Horn Y, Xu ZJ. Cations in octahedral sites: A descriptor for oxygen electrocatalysis on transition-metal spinels. Adv Mater. 2017;29(23):1606800.[DOI]
-
160. Gao X, Chen Y, Wang Y, Zhao L, Zhao X, Du J, et al. Next-generation green hydrogen: Progress and perspective from electricity, catalyst to electrolyte in electrocatalytic water splitting. Nanomicro Lett. 2024;16(1):237.[DOI]
-
161. Pan J, Zhao W, Zhou Y, Wu J, Cheng W, Shi Y, et al. Conformal electronics: Materials, fabrication, and emerging applications. FlexMat. 2025;2(3):341-364.[DOI]
-
162. Peng S, Gong F, Li L, Yu D, Ji D, Zhang T, et al. Necklace-like multishelled hollow spinel oxides with oxygen vacancies for efficient water electrolysis. J Am Chem Soc. 2018;140(42):13644-13653.[DOI]
-
163. Peng X, Pi C, Zhang X, Li S, Huo K, Chu PK. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustainable Energy Fuels. 2019;3(2):366-381.[DOI]
-
164. Wang Y, Jia J, Zhao X, Hu W, Li H, Bai X, et al. Lowering the coordination of octahedra in spinel oxides by the robust Fe–N bonds for enhancing oxygen evolution reaction. ACS Catal. 2024;14(4):2313-2323.[DOI]
-
165. Huang D, Yu J, Zhang Z, Engtrakul C, Burrell A, Zhou M, et al. Enhancing the electrocatalysis of LiNi0.5Co0.2Mn0.3O2 by introducing lithium deficiency for oxygen evolution reaction. ACS Appl Mater Interfaces. 2020;12(9):10496-10502.[DOI]
-
166. Feng X, Zhai B, Cheng R, Yin L, Wen Y, Jiang J, et al. Phase engineering of 2D spinel-type manganese oxides. Adv Mater. 2023;35(42):2304118.[DOI]
-
167. Gardner GP, Go YB, Robinson DM, Smith PF, Hadermann J, Abakumov A, et al. Structural requirements in lithium cobalt oxides for the catalytic oxidation of water. Angew Chem Int Ed. 2012;51(7):1616-1619.[DOI]
-
168. Xu Q, Yang K, Guo L, Wang S. Diatom biorefinery: Comprehensive resource utilization and economic feasibility for sustainable development. Sustain Eng Novit. 2025;1(1):3.[DOI]
-
169. Wu Y, Tang S, Shi W, Ning Z, Du X, Ye C, et al. Graphene-based phthalocyanine-assembled synergistic Fe-co-Ni trimetallic single-atomic bifunctional electrocatalysts by rational design for boosting oxygen reduction/evolution reactions. Carbon Energy. 2025;(9):e70062.[DOI]
-
170. Varhade S, Tetteh EB, Saddeler S, Schumacher S, Aiyappa HB, Bendt G, et al. Crystal plane-related oxygen-evolution activity of single hexagonal Co3O4 spinel particles. Chemistry A European J. 2023;29(12):e202203474.[DOI]
-
171. Hou Z, Cui C, Li Y, Gao Y, Zhu D, Gu Y, et al. Lattice-strain engineering for heterogenous electrocatalytic oxygen evolution reaction. Adv Mater. 2023;35(39):e2209876.[DOI]
-
172. Wu Z, Xian J, Dai J, Fang G, Fan M, Tian H, et al. Microwave-pulse synthesis of tunable 2D porous nickel-enriched LaMnxNi1−xO3 solid solution for efficient electrocatalytic urea oxidation. J Mater Chem A. 2024;12(12):7047-7057.[DOI]
-
173. Fan M, Tian H, Wu Z, Dai J, Ma X, You Y, et al. Microwave shock synthesis of porous 2D non-layered transition metal carbides for efficient hydrogen evolution. SusMat. 2025;5(2):e252.[DOI]
-
174. Gong N, Yang J, Li W, Sun S. Investigation of wheel-rail wear reduction by using MRF rubber joints with bidirectional adjustable stiffness. Smart Mater Devices. 2025;1:202530.[DOI]
-
175. Zhang T, Fan HJ. Grafted superstructure renders catalyst stability in alkaline water oxidation. cScience. 2025;1:e70000.[DOI]
-
176. Li B, Hu K, Tian Z, Chen L. Multiscale simulation in fuel cell and electrolyzer systems: A review of methods, applications, and future prospects. Sustain Eng Novit. 2025;1(1):5.[DOI]
-
177. Yiliguma , Wang Z, Xu W, Wang Y, Cui X, Al-Enizi AM, et al. Bridged-multi-octahedral cobalt oxide nanocrystals with a co-terminated surface as an oxygen evolution and reduction electrocatalyst. J Mater Chem A. 2017;5(16):7416-7422.[DOI]
-
178. Chen P, Ye J, Wang H, Ouyang L, Zhu M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J Alloys Compd. 2021;883:160833.[DOI]
-
179. Chen X, Niu F, Ma T, Li Q, Wang S, Shen S. Anchoring group regulation in semiconductor/molecular complex hybrid photoelectrode for photoelectrochemical water splitting. Smart Mol. 2025;3(2):e20240056.[DOI]
-
180. Smith RD, Prévot MS, Fagan RD, Zhang Z, Sedach PA, Siu MK, et al. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science. 2013;340(6128):60-63.[DOI]
-
181. Fang G, Liu K, Fan M, Xian J, Wu Z, Wei L, et al. Unveiling the electron configuration-dependent oxygen evolution activity of 2D porous Sr-substituted LaFeO3 perovskite through microwave shock. Carbon Neutralization. 2023;2(6):709-720.[DOI]
-
182. Zhu Y, Tang Z, Yuan L, Li B, Shao Z, Guo W. Beyond conventional structures: Emerging complex metal oxides for efficient oxygen and hydrogen electrocatalysis. Chem Soc Rev. 2025;54(2):1027-1092.[DOI]
-
183. Wang J, Gao Y, Chen D, Liu J, Zhang Z, Shao Z, et al. Water splitting with an enhanced bifunctional double perovskite. ACS Catal. 2018;8(1):364-371.[DOI]
-
184. Wang X, Hu J, Lu T, Wang H, Sun D, Tang Y, et al. Importing atomic rare-earth sites to activate lattice oxygen of spinel oxides for electrocatalytic oxygen evolution. Angew Chem Int Ed. 2025;64(3):e202415306.[DOI]
-
185. Esquius JR, LaGrow AP, Jin H, Yu Z, Araujo A, Marques R, et al. Mixed iridium-nickel oxides supported on antimony-doped tin oxide as highly efficient and stable acidic oxygen evolution catalysts. Mater Futur. 2024;3(1):015102.[DOI]
-
186. Qin S, Dai J, Tian H, Zhang H, Huang J, Guan T, et al. 3D printing driving innovations in extreme low-temperature energy storage. Virtual Phys Prototyp. 2025;20:e2459798.[DOI]
-
187. Wang J, Liu X, Bouwman AF, Vilmin L, Beusen AHW, Mogollón JM, et al. Global inland-water oxygen cycle has changed in the Anthropocene. Sci Adv. 2025;11(14):eadr1695.[DOI]
-
188. Qin S, Dai J, Wang M, Zhang H, Cheng S, Xu W, et al. Unleashing the potential of metastable materials in electrocatalytic water splitting. ACS Mater Lett. 2025;7(2):524-543.[DOI]
-
189. Wang HY, Hung SF, Chen HY, Chan TS, Chen HM, Liu B. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4. J Am Chem Soc. 2016;138(1):36-39.[DOI]
-
190. Qin X, Kim D, Piao Y. Metal-organic frameworks-derived novel nanostructured electrocatalysts for oxygen evolution reaction. Carbon Energy. 2021;3(1):66-100.[DOI]
-
191. Radolinski J, Vremec M, Wachter H, Birk S, Brüggemann N, Herndl M, et al. Drought in a warmer, CO2-rich climate restricts grassland water use and soil water mixing. Science. 2025;387(6731):290-296.[DOI]
-
192. Wei L, Dai J, Qin S, Wang M, Zhu Z, Xu W, et al. Two-dimensional materials for high-current-density seawater electrolysis. Green Chem. 2025;27(29):8755-8776.[DOI]
-
193. Sadeghi E, Chamani S, Peighambardoust NS, Aydemir U. Unveiling the potential of metal diborides for electrocatalytic water splitting: A comprehensive review. Energy Environ Mater. 2025;8(3):e12873.[DOI]
-
194. Schelling PK, Phillpot SR, Wolf D. Mechanism of the cubic-to-tetragonal phase transition in zirconia and yttria-stabilized zirconia by molecular-dynamics simulation. J Am Ceram Soc. 2001;84(7):1609-1619.[DOI]
-
195. Wang B, Tao N, Liu J, Wang H, Du Y, Yang H, et al. Spindle spinel CoFeCoO4 microparticles/rGO as an oxygen reduction and oxygen evolution catalyst. Nano. 2019;14(4):1950043.[DOI]
-
196. Bo S, Zhang X, Wang C, Wang H, Chen X, Zhou W, et al. Inorganic-organic hybrid cobalt spinel oxides for catalyzing the oxygen evolution reaction. Nat Commun. 2025;16(1):2483.[DOI]
-
197. He B, Hosseini P, Escalera-López D, Schulwitz J, Rüdiger O, Hagemann U, et al. Effects of dynamic surface transformation on the activity and stability of mixed co-Mn cubic spinel oxides in the oxygen evolution reaction in alkaline media. Adv Energy Mater. 2025;15(8):2403096.[DOI]
-
198. Li A, Kong S, Guo C, Ooka H, Adachi K, Hashizume D, et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat Catal. 2022;5(2):109-118.[DOI]
-
199. Liu Y, Ying Y, Fei L, Liu Y, Hu Q, Zhang G, et al. Valence engineering via selective atomic substitution on tetrahedral sites in spinel oxide for highly enhanced oxygen evolution catalysis. J Am Chem Soc. 2019;141(20):8136-8145.[DOI]
-
200. Avcı ÖN, Sementa L, Fortunelli A. Mechanisms of the oxygen evolution reaction on NiFe2O4 and CoFe2O4 inverse-spinel oxides. ACS Catal. 2022;12(15):9058-9073.[DOI]
-
201. Zhou Y, Sun S, Wei C, Sun Y, Xi P, Feng Z, et al. Significance of engineering the octahedral units to promote the oxygen evolution reaction of spinel oxides. Adv Mater. 2019;31(41):e1902509.
-
202. Woo H, Kwon T, Prabhakaran S, Lee Y, Kim DH, Kim MH. Rational lattice engineering of spinel CoxRh3–xO4 solid solution expediting oxygen evolution reaction. ACS Sustainable Chem Eng. 2023;11(45):16205-16216.[DOI]
-
203. Zhuang L, Ge L, Yang Y, Li M, Jia Y, Yao X, et al. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv Mater. 2017;29(17):1606793.[DOI]
-
204. Baek J, Hossain MD, Mukherjee P, Lee J, Winther KT, Leem J, et al. Synergistic effects of mixing and strain in high entropy spinel oxides for oxygen evolution reaction. Nat Commun. 2023;14(1):5936.[DOI]
-
205. Sun Y, Liao H, Wang J, Chen B, Sun S, Ong SJH, et al. Covalency competition dominates the water oxidation structure–activity relationship on spinel oxides. Nat Catal. 2020;3(7):554-563.[DOI]
-
206. Maiyalagan T, Jarvis KA, Therese S, Ferreira PJ, Manthiram A. Spinel-type lithium cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen reduction reactions. Nat Commun. 2014;5:3949.[DOI]
-
207. Dutta S, Ray C, Negishi Y, Pal T. Facile synthesis of unique hexagonal nanoplates of Zn/co hydroxy sulfate for efficient electrocatalytic oxygen evolution reaction. ACS Appl Mater Interfaces. 2017;9(9):8134-8141.[DOI]
-
208. Qin Y, Cao B, Zhou XY, Xiao Z, Zhou H, Zhao Z, et al. Orthorhombic (Ru, Mn)2O3: A superior electrocatalyst for acidic oxygen evolution reaction. Nano Energy. 2023;115:108727.[DOI]
-
209. Li C, Han X, Cheng F, Hu Y, Chen C, Chen J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat Commun. 2015;6:7345.[DOI]
-
210. Janani G, Surendran S, Choi H, Han MK, Sim U. In situ grown CoMn2O4 3D-tetragons on carbon cloth: Flexible electrodes for efficient rechargeable zinc-air battery powered water splitting systems. Small. 2021;17(47):e2103613.[DOI]
-
211. Sau S, Kundu M, Roy S, Mondal I, Biswas S, Halder P, et al. Bifunctional spinel ZnMn2O4 nanostructures for efficient supercapacitors and water splitting electrocatalysts: A synergistic experimental and modeling study. J Mater Chem A. 2025;13(37):30937-30951.[DOI]
-
212. Yu MQ, Li YH, Yang S, Liu PF, Pan LF, Zhang L, et al. Mn3O4 nano-octahedrons on Ni foam as an efficient three-dimensional oxygen evolution electrocatalyst. J Mater Chem A. 2015;3(27):14101-14104.[DOI]
-
213. Jeghan SMN, Lee G. One-dimensional hierarchical nanostructures of NiCo2O4, NiCo2S4 and NiCo2Se4 with superior electrocatalytic activities toward efficient oxygen evolution reaction. Nanotechnology. 2020;31(29):295405.[DOI]
-
214. Ganguli S, Ghosh S, Tudu G, Koppisetti HVSRM, Mahalingam V. Design principle of monoclinic NiCo2Se4 and Co3Se4 nanoparticles with opposing intrinsic and geometric electrocatalytic activity toward the OER. Inorg Chem. 2021;60(13):9542-9551.[DOI]
-
215. Cheng F, Shen J, Peng B, Pan Y, Tao Z, Chen J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat Chem. 2011;3(1):79-84.[DOI]
-
216. He B, Hosseini P, Priamushko T, Trost O, Budiyanto E, Bondue C, et al. Atomic-scale insights into surface reconstruction and transformation in Co-Cr spinel oxides during the oxygen evolution reaction. Nat Commun. 2025;16(1):9895.[DOI]
-
217. Du J, Pan Y, Zhang T, Han X, Cheng F, Chen J. Facile solvothermal synthesis of CaMn2O4 nanorods for electrochemical oxygen reduction. J Mater Chem. 2012;22(31):15812-15818.[DOI]
-
218. Yamaura K, Huang Q, Zhang L, Takada K, Baba Y, Nagai T, et al. Spinel-to-CaFe2O4-type structural transformation in LiMn2O4 under high pressure. J Am Chem Soc. 2006;128(29):9448-9456.[DOI]
-
219. Zhang J, Zhang Y, Wu X, Ma Y, Chien SY, Guan R, et al. Correlation between structural changes and electrical transport properties of spinel ZnFe2O4 nanoparticles under high pressure. ACS Appl Mater Interfaces. 2018;10(49):42856-42864.[DOI]
-
220. Bahadur A, Hussain W, Iqbal S, Ullah F, Shoaib M, Liu G, et al. A morphology controlled surface sulfurized CoMn2O4 microspike electrocatalyst for water splitting with excellent OER rate for binder-free electrocatalytic oxygen evolution. J Mater Chem A. 2021;9(20):12255-12264.[DOI]
-
221. Talanov MV, Shirokov VB, Avakyan LA, Talanov VM, Borlakov KS. Vanadium clusters formation in geometrically frustrated spinel oxide AlV2O4. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2018;74(4):337-353.[DOI]
Copyright
© The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




