Table of Contents
Benzodiazepine-induced iatrogenic aging: Increased risk and worsened outcomes of age-associated pathologies
The Hippocratic Oath enshrines the ethical imperative primum non nocere -“first, do no harm”- thereby guiding medicine practice toward the meticulous avoidance of interventions that may compromise patients’ physiological integrity and overall ...
More.The Hippocratic Oath enshrines the ethical imperative primum non nocere -“first, do no harm”- thereby guiding medicine practice toward the meticulous avoidance of interventions that may compromise patients’ physiological integrity and overall well-being. Following this principle, benzodiazepines were initially introduced as safer alternatives to barbiturates and have since become one of the most commonly prescribed drug classes for the long-term management of neuropsychiatric disorders in older adults with progressive health deterioration. However, emerging evidence implicates the endogenous benzodiazepine-like peptide, acyl-CoA binding protein/diazepam-binding inhibitor, in the orchestration of maladaptive stress responses. These responses are associated with accelerated pathological aging and increased risks of a spectrum of age-associated morbidities, including metabolic syndrome, cardiovascular diseases, cancers, and immune dysfunction. Corroborating these mechanistic insights, retrospective observational studies have consistently reported significant correlations between long-term benzodiazepine use and elevated risks of cardiovascular mortality, dementia, cancer incidence, impaired responsiveness to immunotherapy, and heightened vulnerability to severe infections. Given these converging lines of evidence, we strongly advocate for the cautious reduction of benzodiazepine prescriptions in elderly patients. Whenever clinically feasible, these agents should be replaced by alternative psychotropic compounds with more favorable risk-benefit profiles, in alignment with contemporary standards of geriatric pharmacotherapy and the ethical imperative to minimize iatrogenic harm.
Less.Léa Montégut, Guido Kroemer
DOI:https://doi.org/10.70401/Geromedicine.2025.0009 - December 08, 2025
Clinical evidence for the use of NAD+ precursors to slow aging
Significant progress in clinical care has extended human life expectancy to unprecedented levels. However, this trend has been parallelled by a rise in years lived with poor health, posing profound challenges not only to individual quality of life, but also ...
More.Significant progress in clinical care has extended human life expectancy to unprecedented levels. However, this trend has been parallelled by a rise in years lived with poor health, posing profound challenges not only to individual quality of life, but also to substantial medical and socioeconomic burdens at the population level. This underscores the urgent need for strategies that extend healthspan alongside lifespan. In this regard, nicotinamide adenine dinucleotide (NAD+) has emerged as a central metabolic cofactor and signaling molecule that regulates processes fundamental to health and longevity, including energy metabolism, mitochondrial function, inflammation, and DNA repair. Importantly, intracellular NAD+ levels decline with age across multiple tissues and organ systems, and restoring NAD+ content has been shown to reinstate cellular and physiological function in various model systems. Among the strategies to augment NAD+, supplementation with its precursors, namely nicotinic acid/niacin, nicotinamide, nicotinamide riboside, and nicotinamide mononucleotide, represents the most practical and extensively studied approach. Over the past two decades, preclinical research and an increasing number of clinical trials have investigated the therapeutic potential of these precursors in preventing or reversing age-associated decline and pathologies. In this review, we synthesize recent clinical advances, critically evaluate the promise and limitations of NAD+ precursor supplementation, and discuss future directions for leveraging NAD+ metabolism to improve healthspan in a rapidly aging global population.
Less.Subhash Khatri, ... Simon Sedej
DOI:https://doi.org/10.70401/Geromedicine.2025.0008 - November 17, 2025
Hallmarks of aging: Integrating molecular and social determinants
The biology of aging is increasingly understood through geroscience frameworks integrating molecular, cellular, physiological, and social hallmarks. Recently, we introduced psychosocial factors including mental illness as an important hallmark of ...
More.The biology of aging is increasingly understood through geroscience frameworks integrating molecular, cellular, physiological, and social hallmarks. Recently, we introduced psychosocial factors including mental illness as an important hallmark of aging. Indeed, exposome-centered approaches reveal complex interactions among socioeconomic, environmental, behavioral, and genomic factors. Precision Geromedicine aims to target all these determinants in a holistic fashion to improve aging trajectories and extend healthspan.
Less.Carlos López-Otín, Guido Kroemer
DOI:https://doi.org/10.70401/Geromedicine.2025.0007 - October 31, 2025
Tau protein isoforms in neuropathological aging: Gerosuppressors, gerogenes or just travel companions
In recent years, the terms “gerosuppressors” and “gerogenes” have been introduced to describe factors that respectively delay or accelerate aging. These factors are present across various cell types. Specific proteins, such as tau predominantly expressed ...
More.In recent years, the terms “gerosuppressors” and “gerogenes” have been introduced to describe factors that respectively delay or accelerate aging. These factors are present across various cell types. Specific proteins, such as tau predominantly expressed in neurons, may act as neuron-specific gerosuppressors or gerogenes. Tau exhibits a dual role influenced by its post-translational modifications, particularly phosphorylation. In this review, we discuss relevant examples of tau isoforms that demonstrate both roles, underscoring its dual influence on neuronal aging.
Less.Jesús Avila, ... José Viña
DOI:https://doi.org/10.70401/Geromedicine.2025.0006 - October 17, 2025
Autophagy in age-related liver disease
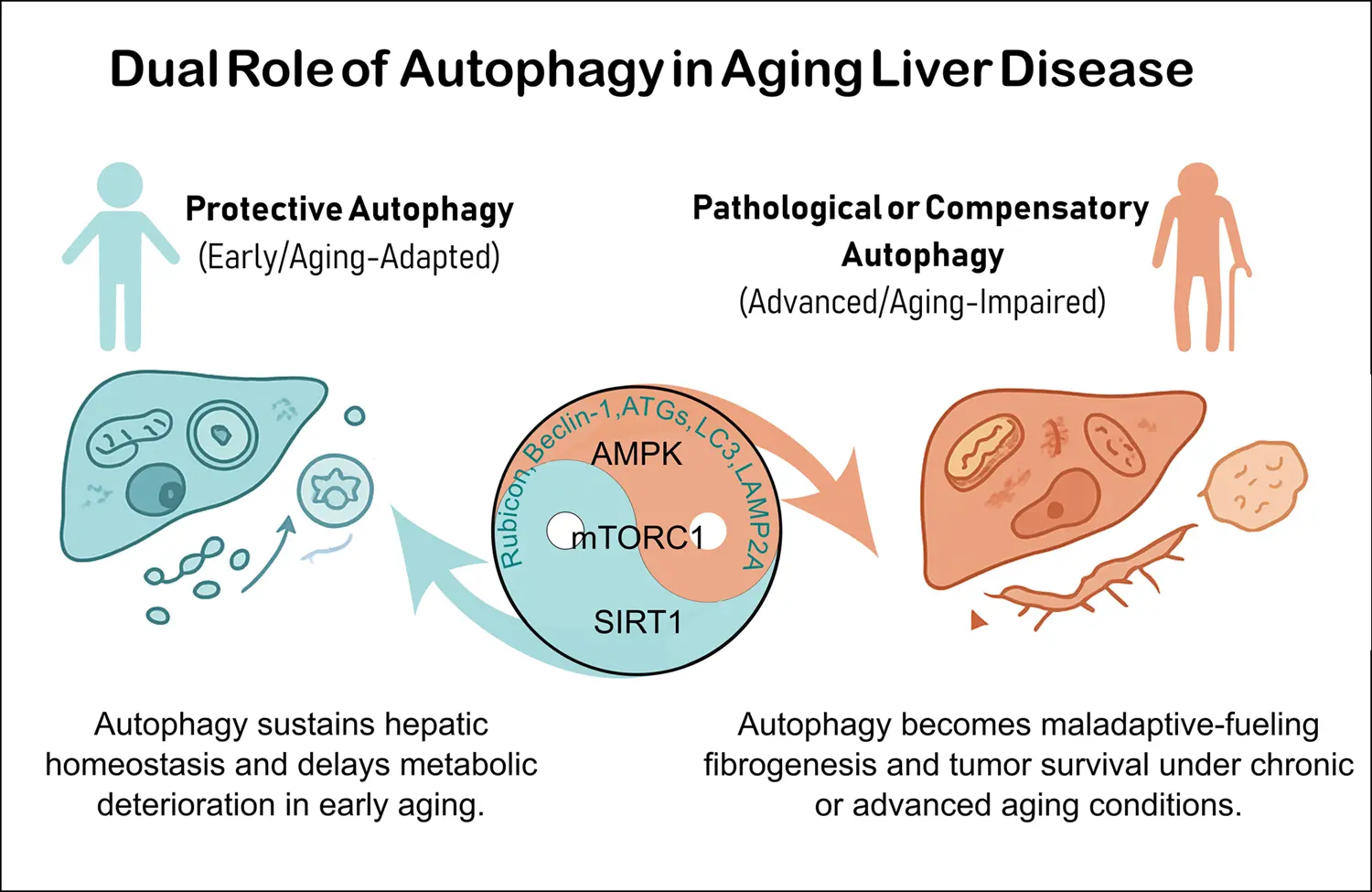
Aging profoundly impacts liver physiology by disrupting autophagy, a lysosome-dependent degradation pathway essential for maintaining cellular homeostasis. Autophagy declines with aging due to reduced expression of core autophagy-related (ATG) genes/proteins, ...
More.Aging profoundly impacts liver physiology by disrupting autophagy, a lysosome-dependent degradation pathway essential for maintaining cellular homeostasis. Autophagy declines with aging due to reduced expression of core autophagy-related (ATG) genes/proteins, defective autophagosome fusion, and impaired selective processes such as lipophagy, mitophagy, and chaperone-mediated autophagy. These alterations contribute to lipid accumulation, oxidative stress, inflammation, and mitochondrial dysfunction, thereby accelerating age-related liver diseases including metabolic-associated fatty liver disease (MAFLD), fibrosis, and hepatocellular carcinoma (HCC). Their molecular mechanisms involve deregulation of nutrient-sensing pathways (mTOR complex 1, AMP-activated protein kinase and sirtuin 1 and 3) and context-dependent roles of autophagy-related proteins (ATG5, ATG7, LC3, Beclin-1, LAMP2A). Importantly, the regulatory role of autophagy differs across disease stages related to liver aging. During early phases, it maintains metabolic balance, mitochondrial quality control, and genomic stability in some diseases such as MAFLD and liver fibrosis. Conversely, in advanced disease, particularly in HCC, persistent autophagy supports tumor cell survival, stemness, and immune evasion. Emerging therapies seek to restore autophagic flux through caloric restriction, physical exercise, caloric restriction mimetics (rapalogs, spermidine, metformin), and pharmacological modulators such as Tat-BECLIN-1 peptides or RUBICON-targeted approaches. However, translating these therapies into clinical practice remains challenging due to systemic effects, stage-specific responses, and lack of reliable non-invasive biomarkers for monitoring autophagy in humans. Advances in nanoparticle-based delivery, biomarker-guided stratification, and combination therapies with tyrosine kinase inhibitors or immune checkpoint inhibitors may offer promising strategies. Overall, precision modulation of autophagy could serve as a potent geroprotective approach to preserve liver function, delay age-related metabolic deterioration, and prevent progression to fibrosis and cancer. Achieving this goal requires considering disease stage, systemic interactions, and autophagy’s context-dependent duality in aging when implementing these strategies.
Less.Roberto Palacios-Ramírez, ... Omar Motiño García-Miguel
DOI:https://doi.org/10.70401/Geromedicine.2025.0005 - October 17, 2025
Education in Healthy Longevity as a prerequisite for a new healthcare model
Hans J. Meij, ... Guido Kroemer
DOI:https://doi.org/10.70401/Geromedicine.2025.0004 - September 30, 2025
The vocabulary of geromedicine: gerovocabulary
Guido Kroemer, ... Andrea B. Maier
DOI:https://doi.org/10.70401/Geromedicine.2025.0002 - May 07, 2025
Geromedicine: A new journal for the clinical application of geroscience
Guido Kroemer, ... Andrea B. Maier
DOI:https://doi.org/10.70401/Geromedicine.2025.0001 - May 07, 2025