Table of Contents
Beyond ferroptosis: Role of GPX4 in osteoarthritis and its therapeutic implications
Osteoarthritis (OA) is increasingly regarded as a whole-organ disease that includes different subsets of joint pathological conditions with variable genetic, biochemical and clinical characteristics. The pathogenesis of OA is perplexing, and disease-modifying ...
More.Osteoarthritis (OA) is increasingly regarded as a whole-organ disease that includes different subsets of joint pathological conditions with variable genetic, biochemical and clinical characteristics. The pathogenesis of OA is perplexing, and disease-modifying drugs are still lacking. Glutathione peroxidase 4 (GPX4), recently best known as the key regulator of ferroptosis implicated in many diseases, including OA, actually has long been identified and reportedly possesses multiple significant biological functions. However, the relationship between GPX4 and OA remains to be elucidated. In this review, we first summarize the current knowledge of GPX4 as a selenoenzyme and the regulation of its expression. Then we scrutinize various possible patterns of involvement of GPX4 in OA. Finally, we also underscore the potential implications and prospects of GPX4-based therapeutic regimens for OA.
Less.Junchen He, ... Kai Sun
DOI:https://doi.org/10.70401/fos.2026.0013 - January 09, 2026
The coming decade in ferroptosis research: Five riddles
Ferroptosis is, in many ways, the odd one out among cell death modalities. It does not, at least as far as we know, require an activating signal. Instead, it represents a default cellular fate that is continuously repressed by a multilayered network of surveillance ...
More.Ferroptosis is, in many ways, the odd one out among cell death modalities. It does not, at least as far as we know, require an activating signal. Instead, it represents a default cellular fate that is continuously repressed by a multilayered network of surveillance systems. At its core, ferroptosis is driven by the unchecked peroxidation of polyunsaturated phospholipids (PUFA-PLs), a vulnerability shaped by lipid bilayer composition. Glutathione peroxidase 4 (GPX4) is a central defense enzyme that reduces lipid hydroperoxides to their corresponding alcohols using glutathione as a cofactor. This is complemented by ferroptosis suppressor protein-1 (FSP1)-mediated regeneration of coenzyme Q10 or vitamin K at the plasma membrane and reinforced by dietary or endogenous radical-trapping antioxidants, such as vitamin E, squalene, and 7-dehydrocholesterol. Still, ferroptosis sensitivity is not just a function of antioxidant failure but also a direct consequence of the architecture of the membrane itself: the abundance of PUFA-PLs, shaped by acyl-CoA synthetases like ACSL4 and others; the relative scarcity or abundance of monounsaturated fatty acids, which confer resistance; the regulation of membrane repair and remodeling enzymes; and the delicate balance of redox-active iron within organelles such as lysosomes. Together, these elements converge to determine whether ferroptosis remains a manageable threat or becomes lethal. Despite growing mechanistic insights, fundamental riddles endure: Why does ferroptosis exist at all? What is the precise role of iron: catalyst, signal, or inherent peril? Where, within the cell or organism, does ferroptosis ignite? Can we safely harness this pathway for clinical benefit? And ultimately, is ferroptosis truly a form of regulated cell death, or the mere emergence of a primordial biochemical vulnerability? Inspired by Douglas Green’s iconic riddle framework, this review distils five unresolved questions that may define the coming decade of ferroptosis research. Rather than solving them, we aim to refine their silhouettes at the intersection of lipid (bio)chemistry, evolutionary biology, and translational opportunity.
Less.Anastasia Levkina, ... Marcus Conrad
DOI:https://doi.org/10.70401/fos.2026.0012 - January 06, 2026
Revisiting the role of iron in ferroptosis
Iron occupies a paradoxical position in biology: indispensable for life through enabling electron transport in metabolism, yet equally capable of driving cellular death. This is exemplified when ferroptosis was defined in 2012 by Dixon and Stockwell (built ...
More.Iron occupies a paradoxical position in biology: indispensable for life through enabling electron transport in metabolism, yet equally capable of driving cellular death. This is exemplified when ferroptosis was defined in 2012 by Dixon and Stockwell (built on parallel work by Marcus Conrad) as a distinct form of regulated cell death. Crucially, ferroptosis is not simply cell death caused by iron poisoning (i.e., iron overload); rather, the ‘dependency of iron’ is evidenced by specific iron chelators that inhibit the induction of death by agents that disrupt cellular redox control (e.g. erastin, which depletes cellular glutathione, and RSL3, which inhibits GPX4 activity, and others). Still, the etymological emphasis on iron does not fully capture the complexity of ferroptosis, which involves a network of potentially lethal metabolic processes encompassing lipids, thiols, and reactive oxygen species. Adding to this tension, recent negative clinical trials of iron chelators in degenerative diseases where ferroptosis has been implicated have tempered enthusiasm for iron-focused strategies and prompted a re-evaluation of iron’s true role in these diseases, and by extension, ferroptosis. In this perspective, we examine the evolving understanding of the ferrum in ferroptosis, its place within the broader metabolic landscape, and its role in neurodegenerative diseases.
Less.Francesca Alves, ... Scott Ayton
DOI:https://doi.org/10.70401/fos.2025.0009 - December 31, 2025
Best practices for cysteine analysis
Accurate measurement of cysteine and related thiol-containing metabolites is essential for understanding cellular redox regulation. However, the intrinsic reactivity and instability of cysteine present substantial analytical challenges. This review ...
More.Accurate measurement of cysteine and related thiol-containing metabolites is essential for understanding cellular redox regulation. However, the intrinsic reactivity and instability of cysteine present substantial analytical challenges. This review summarizes the biochemical context of cysteine and glutathione metabolism, emphasizing their dynamic redox equilibria and physiological relevance. We critically examine existing analytical approaches, including mass spectrometry-based, enzyme-coupled, and colorimetric methods, and discuss their respective strengths and limitations. Particular attention is given to sample preparation, derivatization strategies, and reagent selection, as these steps are crucial for preserving native thiol-disulfide status. Among various alkylating agents, N-ethylmaleimide is identified as the most reliable for thiol stabilization in liquid chromatography–mass spectrometry (LC-MS) workflows, while specific reagents such as monobromobimane or β-(4-hydroxyphenyl)ethyl iodoacetamide (HPE-IAM) are required for persulfide and polysulfide detection. The review also highlights the pitfalls of using indirect surrogates—such as glutathione or cystathionine levels—to infer cysteine availability, which can lead to significant misinterpretation of metabolic states. We conclude that direct LC-MS-based quantification of cysteine and glutathione, combined with careful derivatization and sample handling, remains the most reliable and accurate approach currently available for the assessment of thiol metabolism and redox homeostasis.
Less.Feroza K. Choudhury, Gina M. DeNicola
DOI:https://doi.org/10.70401/fos.2025.0010 - December 31, 2025
Targeting ferroptosis pathways in cancer: Emerging molecular targets and therapeutic strategies
Ferroptosis, a regulated form of cell death driven by iron-dependent lipid peroxidation, has emerged as a crucial tumor suppressive mechanism and a promising therapeutic target in oncology. This review synthesizes the current understanding of its core molecular ...
More.Ferroptosis, a regulated form of cell death driven by iron-dependent lipid peroxidation, has emerged as a crucial tumor suppressive mechanism and a promising therapeutic target in oncology. This review synthesizes the current understanding of its core molecular machinery, encompassing lipid metabolism, iron homeostasis, and multi-layered cellular defense systems. We highlight the unique metabolic and genetic vulnerabilities that render specific cancer cell types intrinsically susceptible to ferroptosis. Furthermore, we discuss the dynamic propagation of ferroptotic signals within the tumor microenvironment and their complex immunomodulatory effects. Central to this review is a strategic framework for targeting ferroptosis, synthesizing recent advances in the development of specific ferroptosis inducers and evaluating their synergistic potential when combined with chemotherapy, radiotherapy, targeted therapy, and immunotherapy. By integrating mechanistic insight with translational perspectives, this work provides a systematic guide for rationally exploiting ferroptosis in cancer treatment.
Less.Bo Zhan, ... Xiao-Feng Zhu
DOI:https://doi.org/10.70401/fos.2025.0011 - December 31, 2025
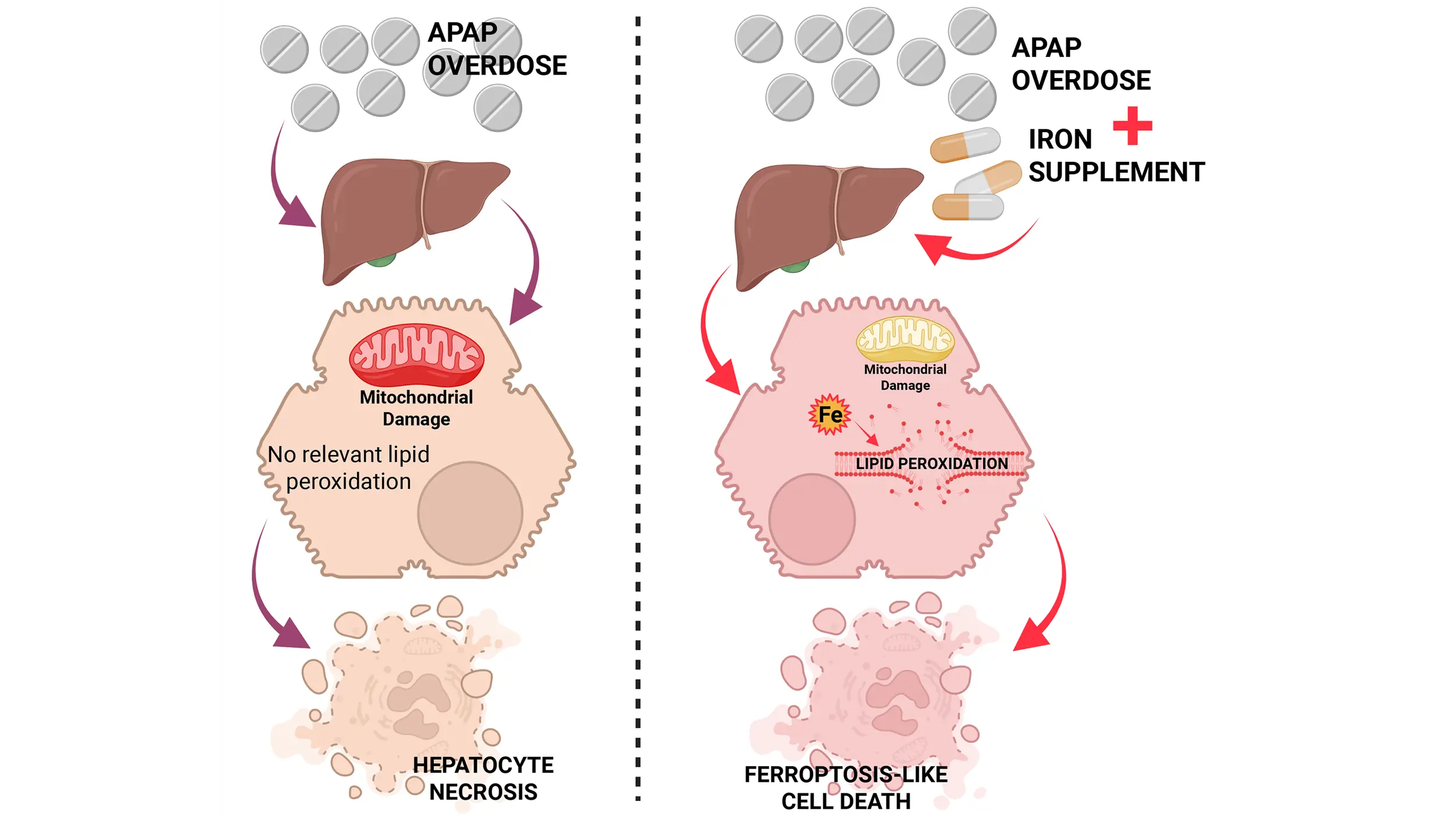
Reactive oxygen species and peroxynitrite in acetaminophen-induced liver injury: Lipid peroxidation and ferroptosis-like cell death
Acetaminophen (APAP) overdose is a clinically relevant model of drug hepatotoxicity and acute liver failure. After decades of research, many aspects of the mechanism of APAP-induced liver injury are well established. These include the cytochrome P450 2E1-mediated ...
More.Acetaminophen (APAP) overdose is a clinically relevant model of drug hepatotoxicity and acute liver failure. After decades of research, many aspects of the mechanism of APAP-induced liver injury are well established. These include the cytochrome P450 2E1-mediated formation of a reactive metabolite, hepatic glutathione depletion, mitochondrial protein adduct formation with oxidant stress and peroxynitrite formation, iron-catalyzed protein nitration in mitochondria, the opening of the mitochondrial permeability transition pore, and release of mitochondrial intermembrane proteins including endonuclease G, which translocate to the nucleus and cause DNA fragmentation, the final step of cell necrosis signaling. However, the mode of cell death remains controversial, as there are many overlaps with apoptosis, necroptosis, and pyroptosis. More recently, ferroptosis has come into focus as a popular cell death mode, creating a new controversial topic. The current review addresses some of the similarities and differences between ferroptosis and APAP-induced necrosis. For example, there is extensive glutathione depletion, but glutathione peroxidase 4 activity is not impaired; there is oxidant stress, but superoxide is used to form peroxynitrite; and there is evidence for an important role of ferrous iron as a catalyst for protein nitration. Moreover, lipid peroxidation is very limited, and excess Vitamin E does not protect. However, cotreatment of an APAP overdose with exogenous ferrous iron can induce extensive lipid peroxidation and switch the mode of cell death. Thus, APAP hepatotoxicity does not involve ferroptosis under normal, clinically relevant conditions, but a change in co-ingested supplements can trigger a switch to ferroptosis-like cell death.
Less.Hartmut Jaeschke, Anup Ramachandran
DOI:https://doi.org/10.70401/fos.2025.0007 - December 19, 2025
Integrating iron and lipid biology in Alzheimer’s disease
Neurons exist at the intersection of two essential yet hazardous metabolic demands: iron-driven bioenergetics and lipid-dependent membrane remodeling. Their reliance on mitochondrial iron for adenosine triphosphate (ATP) generation, combined with ...
More.Neurons exist at the intersection of two essential yet hazardous metabolic demands: iron-driven bioenergetics and lipid-dependent membrane remodeling. Their reliance on mitochondrial iron for adenosine triphosphate (ATP) generation, combined with the requirement for polyunsaturated fatty acids to sustain synaptic plasticity, creates a biochemical environment primed for ferroptosis. This Perspective examines how the interplay between iron and lipid metabolism defines neuronal vulnerability in neurodegenerative diseases, focusing on apolipoprotein E (ApoE) as a metabolic gatekeeper coordinating those pathways. Beyond its canonical function in lipid transport, ApoE acts as a potent anti-ferroptotic factor by inhibiting ferritinophagy and restraining the release of labile iron, thereby coupling lipid trafficking with iron homeostasis. Dysregulation of this axis in Alzheimer’s disease amplifies lipid peroxidation and compromises antioxidant defenses. Parallel mechanisms are observed in Neurodegeneration with Brain Iron Accumulation disorders, where mutations in lipid-metabolic genes paradoxically lead to brain iron accumulation, underscoring the genetic entanglement of these pathways. Collectively, these findings support a unifying model in which neuronal ferroptosis arises not from isolated iron overload or lipid imbalance, but from the breakdown of a coordinated iron-lipid defense network. We propose that restoring this equilibrium through modulation of ApoE function, preservation of mitochondrial iron utilization, and suppression of lipid peroxidation represents a promising avenue for therapeutic intervention in neurodegenerative disease.
Less.Abdel Ali Belaidi
DOI:https://doi.org/10.70401/fos.2025.0006 - December 17, 2025