Table of Contents
SpanAttNet: A hybrid SpanConv SPDConv architecture with residual self attention for viral protein subcellular localization
Aims: The subcellular localization of viral proteins can give insight into virus replication, immune evasion, and the development of therapeutic targets. Traditional experimental methods for determining localization are time-consuming and costly ...
More.Aims: The subcellular localization of viral proteins can give insight into virus replication, immune evasion, and the development of therapeutic targets. Traditional experimental methods for determining localization are time-consuming and costly to perform, which calls for robust computational approaches. In this paper, we propose designing a computational method for identifying the subcellular localization of viral proteins.
Methods: In the effort to improve feature extraction for viral protein subcellular localization, a novel hybrid deep learning architecture, SpanAttNet, was proposed by incorporating span-based convolution with spatial pyramid dilated convolution and a residual self-attention mechanism. Three commonly used sequence descriptors, AAC, PseAAC, and DDE, each combined with PCA for feature dimension reduction, were systematically used to benchmark SpanAttNet.
Results: Among the individual descriptors, the best performance was yielded by PseAAC (accuracy 93.95%, MCC 91.18% at ρ = 0.8 PCA reduction), while optimal performance from DDE was at minimum reduction (accuracy 87.00% at ρ = 0.2). Moreover, ensemble feature fusion across the various descriptors elevated SpanAttNet to its top performance, reaching an MCC of 93.79% and an F1-score of 92.91%, hence achieving the best balance between sensitivity and specificity. Compared to state-of-the-art models, SpanAttNet managed to consistently match or surpass predictive accuracy, demonstrating strong generalizability.
Conclusion: We establish SpanAttNet as a robust and biologically informed predictor for viral protein subcellular localization, with strong potential for extension to multi-label classification and broader proteomic applications.
Less.Grace-Mercure Bakanina Kissanga, ... Hao Lin
DOI:https://doi.org/10.70401/cbm.2025.0006 - December 31, 2025
A survey of deep learning methods for drug-drug interaction prediction
Drug-Drug Interaction (DDI) prediction plays a critical role in ensuring clinical medication safety and optimizing therapeutic regimens, while also representing a significant challenge in the drug development process. With the rapid advancement of artificial ...
More.Drug-Drug Interaction (DDI) prediction plays a critical role in ensuring clinical medication safety and optimizing therapeutic regimens, while also representing a significant challenge in the drug development process. With the rapid advancement of artificial intelligence technologies, computational methods based on machine learning and deep learning have emerged as the mainstream paradigm in DDI research. In this survey, we systematically review the latest research progress and establish a clear methodological taxonomy that traces the evolution from classical feature engineering to modern deep learning architectures. Beyond detailing foundational molecular representations, we provide a comprehensive overview of DDI prediction techniques, encompassing sequence-based models, graph-based models, Transformers, and Graph Transformers. Our analysis culminates in a dedicated discussion of emerging advanced strategy paradigms, such as multimodal fusion and specialized pre-training and fine-tuning schemes. Furthermore, we synthesize current challenges with contemporary solutions and discuss their practical implications for clinical decision support, providing a forward-looking perspective for the continued development of DDI prediction models.
Less.Xu Guo, ... Leyi Wei
DOI:https://doi.org/10.70401/cbm.2025.0005 - December 31, 2025
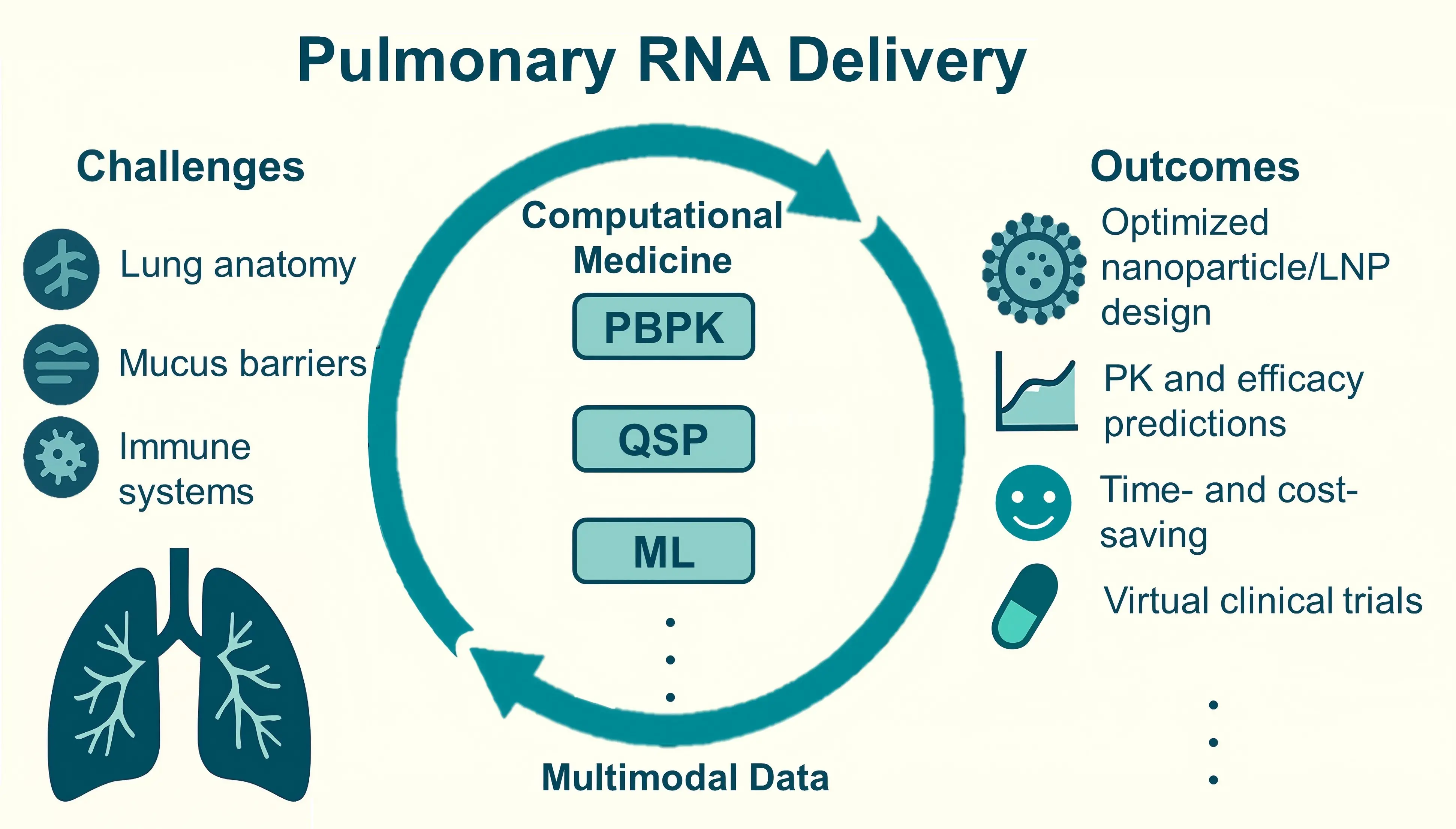
Computational approach to pulmonary delivery of therapeutical RNAs
Targeted delivery of RNA-based therapeutics to the lungs remains a substantial challenge due to the unique anatomy of lung tissue and its complex immune barriers. In recent years, the convergence of physiologically based pharmacokinetic (PBPK) models, quantitative ...
More.Targeted delivery of RNA-based therapeutics to the lungs remains a substantial challenge due to the unique anatomy of lung tissue and its complex immune barriers. In recent years, the convergence of physiologically based pharmacokinetic (PBPK) models, quantitative systems pharmacology approaches, and machine learning algorithms has led to the development of computational medicine frameworks, providing intelligent tools for addressing the aforementioned challenges in efficient pulmonary delivery. By integrating experimental data with predictive computational models, these approaches have advanced the development of RNA therapies for pulmonary diseases. The deep integration of multimodal data is expected to further accelerate drug discovery and clinical translation. In this review, we systematically summarize these computational approaches in designing and optimizing pulmonary RNA delivery systems. We particularly highlight the mechanism-based rational design of RNA therapies through simulations and predictions of biodistribution, cellular targeting, and intracellular transport processes.
Less.Xianan Li, ... Pu Chen
DOI:https://doi.org/10.70401/cbm.2025.0004 - November 28, 2025
MediHerb: A multi-modal enhanced framework for disease inference via herbal knowledge
Aims: Development of robust and effective methods for uncovering herb interactions and constructing herb–disease associations requires the integration of diverse biological and medical information. A key challenge in Traditional Chinese Medicine ...
More.Aims: Development of robust and effective methods for uncovering herb interactions and constructing herb–disease associations requires the integration of diverse biological and medical information. A key challenge in Traditional Chinese Medicine (TCM) research is to robustly uncover herb interactions and construct reliable herb–disease associations. This task requires handling the inherently high-dimensional, multi-label, and cross-domain nature of prescription data. Existing approaches provide limited representation capacity and insufficient integration of biomedical knowledge, restricting their ability to capture the complex semantics underlying the relationships between herbs and diseases.
Methods: To address the limitations of existing approaches, we propose MediHerb, a multi-modal enhanced framework for disease inference via herbal knowledge. MediHerb unifies five complementary modalities: molecular sequences, fingerprints, physicochemical properties, graphical prescription representations, and the description of TCM prescriptions into a shared latent space. An attention-based fusion mechanism aligns the semantics across molecular, herbal, and diagnostic levels, enabling multi-granularity reasoning. To further promote accessibility, a lightweight graphical interface has been developed to support interaction with both the model and open datasets.
Results: Experimental results on benchmark datasets demonstrate that MediHerb substantially outperforms existing baselines in herb–disease inference. Beyond predictive accuracy, the learned embeddings and model attention patterns reveal meaningful biological and pharmacological insights, confirming that MediHerb captures the mechanistic underpinnings of herb–disease associations.
Conclusion: MediHerb highlights the potential of knowledge-enhanced multi-modal fusion to bridge molecular, herbal, and clinical semantics, offering a more interpretable and holistic approach to understanding TCM prescriptions.
Less.Xiaoyi Liu, ... Jijun Tang
DOI:https://doi.org/10.70401/cbm.2025.0003 - November 25, 2025
A comprehensive review on neuropeptides: databases and computational tools
Neuropeptides are crucial signaling molecules that regulate diverse physiological processes spanning growth, social behavior, learning, memory, metabolism, homeostasis, reproduction, and neural differentiation across both nervous and peripheral ...
More.Neuropeptides are crucial signaling molecules that regulate diverse physiological processes spanning growth, social behavior, learning, memory, metabolism, homeostasis, reproduction, and neural differentiation across both nervous and peripheral systems. Dysregulation of neuropeptides signaling is closely linked to various pathological conditions, such as neurological disorders, metabolic diseases, cardiovascular conditions, and even cancer, positioning them as potential therapeutic agents or targets for intervention. In recent years, research into neuropeptides has accelerated, with vast amounts of data continuously accumulating in multiple databases. However, the study of neuropeptides is often impeded by the need for extensive and time-consuming experimental investigations. As a result, computational tools have become essential for the rapid, large-scale identification of neuropeptides. This review systematically discusses neuropeptide-related databases and computational tools. These databases organize extensive data on neuropeptide sequences, structures, and functions. Among these, NeuroPep2.0, with 11,417 neuropeptide entries, is currently the most widely used dataset for neuropeptide prediction. Additionally, this review explores the application of computational approaches in neuropeptide prediction. While early methods predominantly relied on homologous sequence alignment and biochemical feature statistics, recent advances in machine learning have significantly enhanced prediction accuracy and efficiency. Tools such as NeuroPred-PLM and DeepNeuropePred, developed by our research group using protein language models, have substantially improved prediction performance. In conclusion, this review provides a comprehensive overview of current neuropeptide databases and computational tools, offering researchers a thorough survey of available resources and analytical methods, and emphasizing the necessity of continuous optimization to advance neuropeptide research and its therapeutic applications.
Less.Wei Xu, ... Yan Wang
DOI:https://doi.org/10.70401/cbm.2025.0001 - April 10, 2025